Keeping up with cancer immunotherapies
By Elizabeth Kirchner, CNP, and Cassandra Calabrese, DO
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
In 2011 the anticytotoxic T-lymphocyte antigen 4 antibody ipilimumab was approved by the FDA for the treatment of melanoma. This represented the first approval of a checkpoint inhibitor, also called cancer immunotherapy. Several more approvals for other agents followed, and six short years later another checkpoint inhibitor, pembrolizumab, was granted the first-ever “agnostic” (i.e., based on a biomarker rather than a specific cancer diagnosis) approval for cancer treatment.
Overall, immunotherapies have revolutionized the treatment of cancer. Like all revolutions, however, this one has not been without collateral damage. Checkpoint inhibitors are associated with a host of immune-related side effects, including rheumatic. Education of rheumatology providers on cancer immunotherapy and immune-related adverse events (IRAEs) has scrambled to keep up and has presented many challenges.
There are currently seven approved checkpoint inhibitors in the United States: atezolizumab, avelumab, durvalumab, ipilimumab, nivolumab, pembrolizumab and most recently cemiplimab (approved Sept. 28, 2018). While they belong to three different classes (anti-PD-1, anti-PD-L1 and anti-CTLA4), in the broadest sense they all work by “taking the foot off the brakes of the immune system.”
Unfortunately, unleashing the immune system allows it to not only battle malignancies, it also allows autoimmune diseases to flourish. Every organ system — from dermatologic to ophthalmologic, gastrointestinal, endocrine, rheumatologic and others — is affected by the side effects of cancer immunotherapy.
Advertisement
The rheumatic IRAEs most often seen are arthralgia, PMR-like illnesses and inflammatory arthritis. Patients with previously diagnosed IMIDs as well as patients with no history of autoimmune disease may be affected, as patients with pre-existing rheumatic diseases often experience flares in the setting of checkpoint inhibitor therapy.
While some patients respond to glucocorticoids, others require intensification of treatment with cDMARDs and/or bDMARDs. Trying to balance the risks and benefits of DMARDs with a current cancer diagnosis is one of the challenges faced by rheumatology providers. Another is that there are many unknowns in terms of how immunosuppression affects immunotherapy. Finally, in the vast majority of non-rheumatic IRAEs the events are self-limited and resolve when immunotherapy is held. Rheumatic IRAEs, in contrast, often persist despite discontinuation of checkpoint inhibitors.
Long-term management of these patients requires multidisciplinary care that adequately addresses the patient’s IMID while treating their cancer.
There are over 20 abstracts at the 2018 ACR Annual Meeting addressing IRAEs from cancer immunotherapy. We will be presenting two abstracts: a case series and systemic review of PMR secondary to checkpoint inhibitors, and will be sharing our experience with a novel IRAE tumor board and its impact on interprofessional clinical confidence and collaboration.
In addition, ACR and ARHP are co-hosting a pre-meeting course on immunology breakthroughs that will include a case study presented by Elizabeth Kirchner and an overview of IRAEs presented by Anne Bass, MD, of the Hospital for Special Surgery. Leonard Calabrese, DO, Director of Cleveland Clinic’s R.J. Fasenmyer Center for Clinical Immunology, will present a plenary session on translational implications of IRAEs.
Advertisement
The plethora of cancer immunotherapy offerings at this year’s Congress illustrates how the rheumatology community is coming together to provide education on this very important and fast-moving topic.
Ms. Kirchner is a nurse practitioner and Dr. Calabrese is a staff physician in the Department of Rheumatic and Immunologic Diseases at Cleveland Clinic.
Advertisement
Advertisement

First-of-its-kind research investigates the viability of standard screening to reduce the burden of late-stage cancer diagnoses

Global R&D efforts expanding first-line and relapse therapy options for patients
Study demonstrates ability to reduce patients’ reliance on phlebotomies to stabilize hematocrit levels

A case study on the value of access to novel therapies through clinical trials

Findings highlight an association between obesity and an increased incidence of moderate-severe disease
Cleveland Clinic Cancer Institute takes multi-faceted approach to increasing clinical trial access 23456
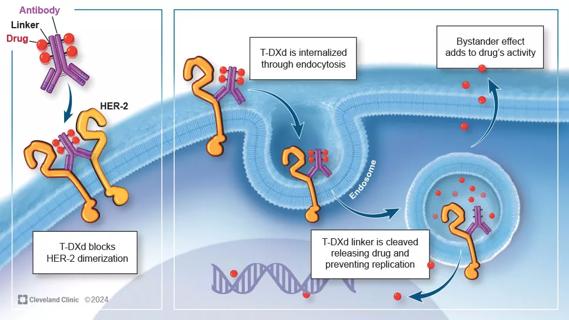
Key learnings from DESTINY trials

Overall survival in patients treated since 2008 is nearly 20% higher than in earlier patients