Pembrolizumab stabilizes cancer in some patients

Standard treatment for metastatic small cell lung cancer (SCLC) has not changed for more than 30 years, and survival rates are poor, with most patients living less than a year after diagnosis.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
The search for better treatment options has led oncologists to consider immune checkpoint inhibitors, which work by encouraging the immune system to attack cancer cells.
“Because checkpoint inhibitors have been so promising and have changed the standard treatment for so many types of cancer, the hope is they could also make an impact on small cell lung cancer,” says Nathan Pennell, MD, PhD, Director of the lung cancer medical oncology program at Cleveland Clinic.
Dr. Pennell recently joined with oncologists from research institutions around the country to conduct a phase 2 maintenance study using the checkpoint inhibitor pembrolizumab to treat patients with extensive-stage small cell lung cancer (ES-SCLC).
The study — which was accepted for an oral presentation at the 2017 American Society of Clinical Oncology (ASCO) Annual Meeting in Chicago — involved 45 patients of which 35 had measurable disease and 10 had brain metastases.
To qualify, patients had to have first received the standard treatment of chemotherapy and then have gone into remission. The median time from the end of chemotherapy to the start of pembrolizumab was five weeks. Patients were treated with pembrolizumab 200 mg intravenously every three weeks. The median number of pembrolizumab cycles was four.
The disease control rate with maintenance pembrolizumab was 42 percent. At the median follow up of six months, the median progression free survival (PFS) was 1.4 months while the irPFS —calculated using immune-related response criteria developed for immunotherapy treatment — was 4.7 months. The median overall survival (OS) was 9.2 months.
Advertisement
The PFS rate was disappointing, but overall the study was not, Dr. Pennell says. “There were clearly a number of people who seemed to be benefitting from the pembrolizumab. Forty-two percent of people who received it had at least stabilization of their cancer and four actually had a further response beyond what they’d gotten with chemotherapy. And the overall survival was 9.2 months, which is quite impressive for metastatic lung cancer patients who are in this setting.”
PD-L1 is a biomarker for non-small cell lung cancer and some studies have shown that patients need to have PD-L1 expression in order to benefit from pembrolizumab. In this trial, however, only one patient showed expression of PD-L1 but many more appeared to get some benefit from the drug.
“So it’s not clear in our trial or in other trials of small cell lung cancer that you need PDL-1 expression in order to benefit from these drugs,” Dr. Pennell says, adding that finding an accurate biomarker is a priority.
“I think the study suggests there are some small cell lung cancer patients that are benefiting from pembrolizumab. It’s just that we don’t have a good way of determining ahead of time who those patients are.”
Advertisement
Advertisement

First-of-its-kind research investigates the viability of standard screening to reduce the burden of late-stage cancer diagnoses

Global R&D efforts expanding first-line and relapse therapy options for patients
Study demonstrates ability to reduce patients’ reliance on phlebotomies to stabilize hematocrit levels

A case study on the value of access to novel therapies through clinical trials

Findings highlight an association between obesity and an increased incidence of moderate-severe disease
Cleveland Clinic Cancer Institute takes multi-faceted approach to increasing clinical trial access 23456
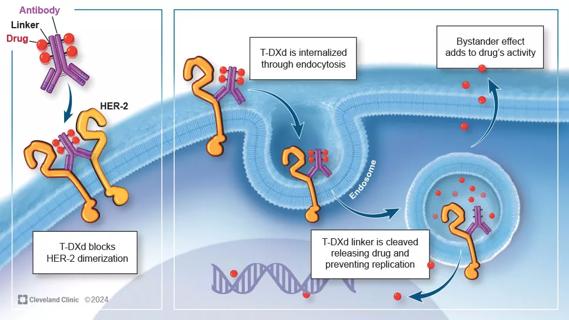
Key learnings from DESTINY trials

Overall survival in patients treated since 2008 is nearly 20% higher than in earlier patients