Urges better attention to patient risk factors for clots.

Among 775 cancer patients in five academic medical centers enrolled in a study on the use of thromboprophylaxis, nearly one-third had contraindications to pharmacologic prophylaxis.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
However, the study, “Pattern of Frequent But Non-Targeted Pharmacologic Thromboprophylaxis (PT) for Hospitalized Patients With Cancer at Academic Medical Centers” found that PT in hospitalized patients with cancer is commonly prescribed without regard to the presence or absence of concomitant risk factors for venous thromboembolic events (VTEs).
VTEs are the second leading cause of death in patients with cancer.
“What’s known is that cancer patients in the hospital are at risk for getting blood clots,” says Alok Khorana, MD, Section Head of Cleveland Clinic’s Gastrointestinal Cancer program.
“And blood clots are important because they can lead to deaths in the hospital,” he says. “Although there is a high known risk in cancer patients, there is a lot of variation amongst patients. There are people who come into the hospital for one day, maybe for chemotherapy or a minor procedure. Probably, the risk (of VTE) for those patients is low.
“There are other patients who are very sick, immobile, have an infection or are on a bunch of different thrombogenic agents, and in those patients the risk is very high.”
While the study headed by Dr. Khorana acknowledges that randomized studies have demonstrated the benefit of pharmacologic thromboprophylaxis in acutely ill hospitalized patients and its use is widely advocated and considered standard practice, several population-based studies indicate that the compliance rates of VTE PT in patients with cancer are low – actually lower than in other high-risk patients who do not have cancer.
Advertisement
Concurrently, PT is being administered too frequently to patients with contraindications for it, an equally challenging potential problem.
The study says “relative contraindications” to PT in hospitalized patients with cancer include: evidence of significant thrombocytopenia (platelet count less than 50,000); active hemorrhage; otherwise considered high-risk for hemorrhage (excluding thrombocytopenia); history of hemorrhage; patient refusal; thromboprophylaxis not within goals of care (e.g., comfort measures only); and heparin allergy or heparin-induced thrombocytopenia. Among those categories, 65.2 percent of the study patients considered at risk had evidence of thrombocytopenia.
According to the study, published rates of VTE PT in patients with cancer are largely based on retrospective medical record reviews by using databases of hospitalized patients. “We performed a prospective, multicenter, cross-sectional study to evaluate the use of PT administration and factors influencing this use in hospitalized patients with cancer,” the study says.
Among the group of patients eligible for PT, 71.4 percent were considered high-risk by the Padua Scoring System (PSS), and 78.8 percent of those patients received PT. Twenty-eight percent were low-risk according to the PSS, and 63 percent also received PT.
The strongest predictor of PT was a prior history of VTE. Patients hospitalized with central venous catheters or for cancer directed therapy (chemotherapy or radiation) were less likely to receive PT.
Advertisement
“Often, these decisions are made by the way the health system is set up,” says Dr. Khorana. “The gist (of the study) is we have to have a better system-based approach. My message to colleagues is: 1) It’s an important problem; 2) it’s preventable; and 3) we need to be judicious about preventing [VTEs], so we need to be doing risk assessment and making sure the right patients are getting prophylaxis.”
The study report was written on behalf of the North America Cancer and Thrombosis Study Group.
Advertisement
Advertisement

First-of-its-kind research investigates the viability of standard screening to reduce the burden of late-stage cancer diagnoses

Global R&D efforts expanding first-line and relapse therapy options for patients
Study demonstrates ability to reduce patients’ reliance on phlebotomies to stabilize hematocrit levels

A case study on the value of access to novel therapies through clinical trials

Findings highlight an association between obesity and an increased incidence of moderate-severe disease
Cleveland Clinic Cancer Institute takes multi-faceted approach to increasing clinical trial access 23456
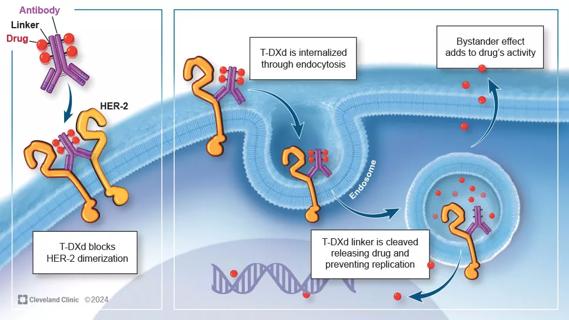
Key learnings from DESTINY trials

Overall survival in patients treated since 2008 is nearly 20% higher than in earlier patients