Recent survey shows more awareness needed
A 2015 randomized phase III trial of newly diagnosed glioblastoma (GBM) patients demonstrated that the combination of Tumor Treating Fields (TTF) with the chemotherapy drug temozolomide was superior to use of temozolomide alone.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
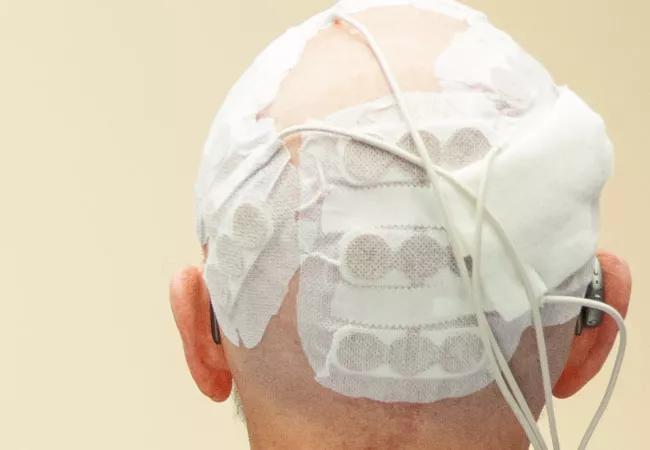
TTF is a noninvasive treatment modality that uses alternating electric fields at specific frequencies and intensities to selectively disrupt mitosis in cancer cells. The trial found that the combination of the two treatment methods increased median overall survival in newly diagnosed GBM by 4.9 months (more than a 30 percent increase in life span).
Recently researchers conducted a survey of radiation oncologists, medical oncologists, neuro-oncologists, neurosurgeons and neurologists to better understand how TTF is used in practices around the United States.
“We were interested in assessing nationwide trends and practice patterns for this treatment regimen,” says John Suh, MD, Chairman of Radiation Oncology at Cleveland Clinic and an author of the study published in the Journal of Neuro-Oncology.
A total of 106 providers responded to the survey. Ninety-five percent were practicing physicians. The most common responders were radiation oncologists (75 percent) and neuro-oncologists (22 percent); medical oncologists (3 percent) and neurosurgeons (1 percent) comprised the remaining responders. A minority (36 percent) were in private practice.
TTF is listed on the current guidelines of the National Comprehensive Cancer Network (NCCN). Of the survey responders, 81 percent were aware of that and 19 percent were not.
In addition, 31 percent of responders reported no TTF-certified physician in their practice. TTF users were more likely to have greater high-grade glioma volume (> 10 GBM patients/year; P = 0.024; relative risk 1.5), be knowledgeable of TTF inclusion on the 2016 NCCN guidelines (P < 0.0001; odds ratio = 10.4; relative risk = 3.0), and specialize in radiation oncology or neuro-oncology (P = 0.016).
Advertisement
Dr. Suh says the results may indicate that some physicians are more comfortable with TTF because it is used more often in their practices. He also says that the treatment, while it extends overall survival, is not for everyone.
“This is a portable device that patients have to wear on their scalp for 18 hours a day,” he says. “Patients need to shave their head to wear the adhesive patches, and some patients may be reluctant to do that. In addition, some physicians do not strongly advocate for its routine use despite the results of the phase III study.”
He adds: “Even at Cleveland Clinic, where we do offer it to newly diagnosed GBM patients who are not on study, less than a third of patients accept this treatment option.”
Nevertheless, Dr. Suh says the survey results indicate that more work needs to be done to inform physicians about TTF and its potential benefits.
Advertisement
Advertisement

First-of-its-kind research investigates the viability of standard screening to reduce the burden of late-stage cancer diagnoses

Global R&D efforts expanding first-line and relapse therapy options for patients

Study demonstrates ability to reduce patients’ reliance on phlebotomies to stabilize hematocrit levels

A case study on the value of access to novel therapies through clinical trials

Findings highlight an association between obesity and an increased incidence of moderate-severe disease

Cleveland Clinic Cancer Institute takes multi-faceted approach to increasing clinical trial access 23456

Key learnings from DESTINY trials

Overall survival in patients treated since 2008 is nearly 20% higher than in earlier patients