Biological potential of disease better informs AS candidacy
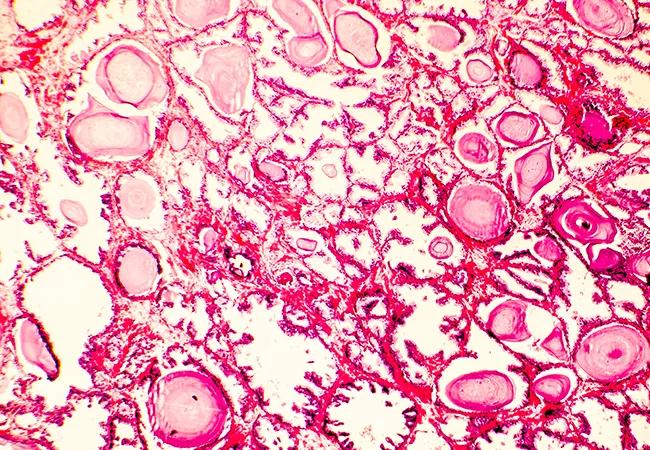
Genomic testing is proving to be a game changer in prostate cancer, particularly in treatment decision-making. A recent Cleveland Clinic study revealed for the first time that the molecular features of a low-grade tumor as measured by genomic testing, rather than the amount of tumor present on biopsy, can identify which cancers should be treated and which are safe to watch.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
The study, led by Eric A. Klein, MD, Chairman, Glickman Urological & Kidney Institute at Cleveland Clinic, identified 296 men with National Comprehensive Cancer Network® (NCCN) very low- and low-risk disease who underwent Oncotype DX® prostate testing between 2013 and 2016 at Cleveland Clinic. The Oncotype DX genomic test reported a Genomic Prostate Score (GPS) that provides a measure of the aggressiveness of prostate cancer. The resulting information can be used to personalize treatment based on the underlying biology of the tumor.
The study, Genomic Scores are Independent of Disease Volume in Men with Favorable Risk Prostate Cancer: Implications for Choosing Men for Active Surveillance, published in The Journal of Urology, sought to determine whether the volume of disease found on biopsy correlates with genomic scores, revealing the tumors’ true biologic potential in a way not possible by standard pathological assessment. The results show that genomics could help in appropriately reclassifying patients and add independent predictive value to typical selection criteria for active surveillance (AS).
AS in prostate cancer is a management strategy that monitors low-grade cancers through digital rectal exams, prostate specific antigen (PSA) testing and biopsy. Intervention is not initiated unless testing indicates a cancer has increased in grade and become more aggressive.
The standard criteria for AS is based on biopsy results. In other words, a patient with one or two low-grade (Gleason 3+3) positive core samples typically is referred for AS, while a patient with three or more cores of even low-grade samples would be referred for more aggressive treatment.
Advertisement
But a small subset of low-grade cancers — 5 percent to 10 percent — have molecular features of high-grade cancer.
“Genomic studies have given us for the first time the ability to identify which low-grade cancers actually have molecular features of high-grade cancers and are not candidates for active surveillance,” says Dr. Klein. “These studies show active surveillance decisions ought to be based on genomic tests — a direct test of a tumor’s biology — and not on how much cancer a patient has.
“It’s a refinement in identifying patients who either need treatment or are good candidates for surveillance based on biology as measured by molecular characteristics.”
Dr. Klein says the use of genomic tests, in conjunction with MRI, are increasingly recognized as a valuable adjuncts in cancer diagnosis and treatment decision-making. In fact, NCCN guidelines now endorse considering genomic testing when determining eligibility for AS due to its ability to improve individual risk reclassification when combined with traditional clinical criteria.
Advertisement
Advertisement

First-of-its-kind research investigates the viability of standard screening to reduce the burden of late-stage cancer diagnoses

Global R&D efforts expanding first-line and relapse therapy options for patients
Study demonstrates ability to reduce patients’ reliance on phlebotomies to stabilize hematocrit levels

A case study on the value of access to novel therapies through clinical trials

Findings highlight an association between obesity and an increased incidence of moderate-severe disease
Cleveland Clinic Cancer Institute takes multi-faceted approach to increasing clinical trial access 23456
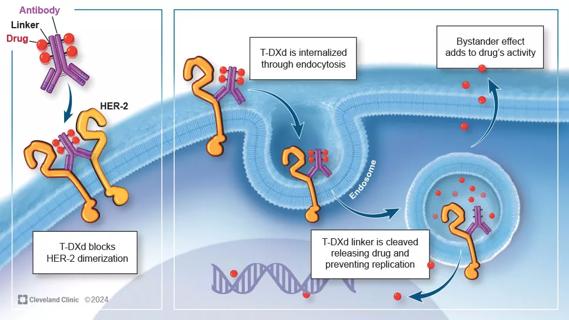
Key learnings from DESTINY trials

Overall survival in patients treated since 2008 is nearly 20% higher than in earlier patients