A New Paradigm

Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
On June 13, 2013, the U.S. Supreme Court made a landmark decision that broke the monopoly on BRCA1 and BRCA2 gene testing held by Myriad Genetics. Testing for BRCA1 and BRCA2 alone and in combination with other genes is now available through several companies.
Traditionally, individuals susceptible to hereditary cancers were screened based on history and offered testing for mutations in a single gene or a set of genes associated with a particular syndrome (e.g., BRCA1 and BRCA2 for hereditary breast and ovarian cancer or mismatch repair genes for Lynch syndrome). Today, advances in next-generation sequencing allow simultaneous testing for multiple genes associated with hereditary cancers.
Multi-gene panel testing encompasses tests for a wide range of mutations, including:
This is a highly cost-effective and time-effective method for the simultaneous evaluation of multiple genes. It has allowed for the identification of 40 to 50 percent more individuals with hereditary cancer gene mutations than is possible testing for BRCA1 and BRCA2 alone. However, these tests have some noteworthy limitations.
Advertisement
Women with BRCA1 mutations have a 65 to 85 percent risk for breast cancer and a 39 to 46 percent risk for ovarian, fallopian tube and primary peritoneal cancers by age 70.
Similarly, women with BRCA2 mutations have a 45 to 85 percent risk of breast cancer and a 10 to 27 percent risk of ovarian cancer by age 70.
Mutations in other genes, including BRIP1, RAD51D and RAD51C, have been described to be associated with a 10 to 15 percent lifetime risk of ovarian cancer.
Approximately 20 to 25 percent of ovarian carcinomas are thought to result from a hereditary predisposition. While BRCA1 and BRCA2 genes are responsible for the vast majority of hereditary ovarian cancers, several other genes within the HR pathway have been shown to contribute to a similar phenotype. Patients with these phenotypes have a better prognosis and clinical response to platinum therapies. In addition, they are likely to benefit from targeted therapies (PARP inhibitors) that exploit defects in the HR pathway.
Current guidelines issued by the National Comprehensive Cancer Network and the Society of Gynecologic Oncologists recommend genetic counseling and testing for all women with ovarian cancer, regardless of age or family history.
In addition, patients with a history consistent with hereditary breast and ovarian cancer should be given the option of pursuing panel testing when other cancer types are present in the family, rare syndromes are being considered or the results would influence medical management.
Advertisement
Advertisement

First-of-its-kind research investigates the viability of standard screening to reduce the burden of late-stage cancer diagnoses

Global R&D efforts expanding first-line and relapse therapy options for patients
Study demonstrates ability to reduce patients’ reliance on phlebotomies to stabilize hematocrit levels

A case study on the value of access to novel therapies through clinical trials

Findings highlight an association between obesity and an increased incidence of moderate-severe disease
Cleveland Clinic Cancer Institute takes multi-faceted approach to increasing clinical trial access 23456
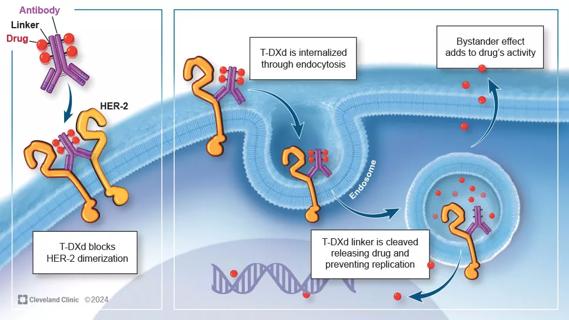
Key learnings from DESTINY trials

Overall survival in patients treated since 2008 is nearly 20% higher than in earlier patients