New treatment option for pre-treated patients

For patients with advanced, previously treated renal cell carcinoma (RCC), cabozantinib is a newer treatment option already shown in a phase 3 clinical trial to improve progression-free survival (PFS) and objective response rate (ORR) compared with everolimus. Now, that trial also meets its secondary endpoint, showing a significant benefit with cabozantinib versus everolimus in median overall survival (OS).
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
“It’s one of the first drugs to show a survival benefit in that setting — along with nivolumab, which was approved last November — adding to the limited therapy options for these patients,” says Brian Rini, MD, oncologist at Cleveland Clinic’s Department of Hematology and Oncology.
METEOR is a registration trial in which 658 previously treated patients were randomized to either cabozantinib or everolimus. Dr. Rini presented the final OS results from METEOR at the 2016 American Society of Clinical Oncology (ASCO) annual meeting in Chicago. The FDA approved cabozantinib for use as second-line treatment for patients with advanced RCC on April 26, 2016, based on these trial results. This follows the agency’s 2012 approval of the drug for the treatment of metastatic medullary thyroid cancer.
The median OS in this trial was 21.4 months for patients receiving cabozantinib versus 16.5 months for everolimus, with a 33 percent reduction in the rate of death (HR 0.67, 95% CI 0.53 to 0.83, P=0.0003). Estimated survival at 18 months was 58 percent for cabozantinib versus 47 percent for everolimus.
No new adverse events were reported, however the drug has its toxicity challenges. Fatigue and diarrhea are the big ones. Fatigue is usually managed with dose reductions, and for diarrhea, supportive medications are used.
“While not toxic in everyone, it’s a balance of risk and benefit, and we will see how it plays out now that it’s available in terms of where people use it, in whom do they use it, and is the toxicity worth the benefit,” says Dr. Rini.
Advertisement
Cabozantinib targets multiple tyrosine kinases involved in RCC, including MET, AXL and three vascular endothelial growth factor (VEGF) receptors. Survival benefit was consistently observed across the pre-specified subgroups, which included the number and type of previous VEGFR tyrosine kinase inhibitors (TKIs) and tumor MET expression levels, as well as prior anti-PD-1/PD-L1 therapy, MSKCC risk group, location and extent of tumor metastases.
While not conclusive evidence, the longer PFS and OS with cabozantinib seen in this trial are supportive of the idea that targeting several TKIs may help delay the usual drug resistance for which RCC is notorious.
What remains to be determined is who might benefit most from this treatment. Dr Rini notes that there are no biomarkers that were developed for cabozantinib, and the best treatment sequence to use with these drugs — as well as whether or not combination therapy will be an effective and viable approach — are all things that remain to be seen. However, this is certainly good news in that there is a new option for patients with previously treated advanced RCC.
Advertisement
Advertisement

First-of-its-kind research investigates the viability of standard screening to reduce the burden of late-stage cancer diagnoses

Global R&D efforts expanding first-line and relapse therapy options for patients
Study demonstrates ability to reduce patients’ reliance on phlebotomies to stabilize hematocrit levels

A case study on the value of access to novel therapies through clinical trials

Findings highlight an association between obesity and an increased incidence of moderate-severe disease
Cleveland Clinic Cancer Institute takes multi-faceted approach to increasing clinical trial access 23456
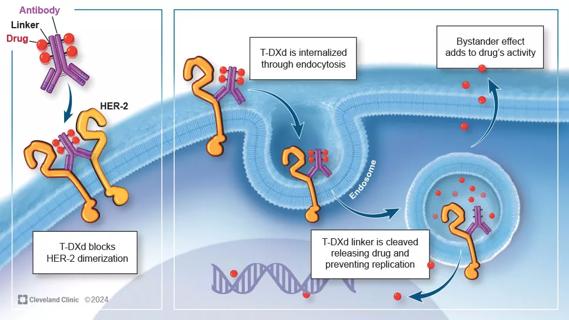
Key learnings from DESTINY trials

Overall survival in patients treated since 2008 is nearly 20% higher than in earlier patients