New recommendations for second-line therapy

In 2016, ASCO published guidelines to assist in clinical decision making in the initial assessment after diagnosis, first and second-line treatment options, palliative and supportive care, and follow-up of patients with metastatic pancreatic cancer. Now, just two years later, ASCO has revised the guidelines to reflect current data.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
“In 2017, new studies were published that caused us to update the 2016 Metastatic Pancreatic Cancer Guideline in 2018,” says Davendra P.S. Sohal, MD, MPH, Director of the Clinical Genomics Program at Cleveland Clinic’s Taussig Cancer Institute. Dr. Sohal led the ASCO guidelines panel.
The updated guidelines, released earlier this year, offer a more structured and nuanced treatment approach for patients with metastatic pancreatic cancer in the second-line setting, who have experienced disease progression or intolerable toxicity during first-line treatment. The revised guidelines make two new recommendations. They include:
Chemotherapy protocol. The new guidelines recommend 5-fluorouracil in combination with nanoliposomal irinotecan as the preferred second-line chemotherapy regimen. If 5-fluorouracil with nanoliposomal irinotecan is unavailable, 5-fluorouracil plus irinotecan or 5-fluorouracil plus oxaliplatin may be offered.
“We moved the recommendation for 5-fluorouracil plus nanoliposomal irinotecan up and softened the recommendation for 5-fluorouracil plus oxaliplatin because of a study in 2017 showing that oxaliplatin may not provide as much benefit as we previously thought,” Dr. Sohal says.
The nuanced update allows physicians to make more informed treatment decisions when considering the order of preference when using chemotherapy.
“If we can avoid oxaliplatin, patients experience less neuropathy for an improved quality of life,” Dr. Sohal says.
MSI testing and immunotherapy. The second update recommends that all patients considering second-line therapy be offered testing for microsatellite instability (MSI) because of the efficacy of the anti-PD-1 antibody pembrolizumab for MSI-positive tumors.
Advertisement
“Tumors with MSI respond very well to immunotherapy,” Dr. Sohal says.
Although patients with MSI-positive tumors are few, the recommendation is an advance for a small subset of patients.
“Not a lot of patients can benefit from immunotherapy, but at least we know which patients will,” Dr. Sohal says.
Although the median overall survival of patients with metastatic pancreatic cancer remains less than one year, the 2018 ASCO updates make the guidelines a more useful tool for clinicians to potentially improve outcomes.
“Immunotherapy is the biggest advance for patients in the second-line setting,” says Dr. Sohal. “It will be really helpful for patients who qualify for it. The results have been very impressive.”
Advertisement
Advertisement

First-of-its-kind research investigates the viability of standard screening to reduce the burden of late-stage cancer diagnoses

Global R&D efforts expanding first-line and relapse therapy options for patients
Study demonstrates ability to reduce patients’ reliance on phlebotomies to stabilize hematocrit levels

A case study on the value of access to novel therapies through clinical trials

Findings highlight an association between obesity and an increased incidence of moderate-severe disease
Cleveland Clinic Cancer Institute takes multi-faceted approach to increasing clinical trial access 23456
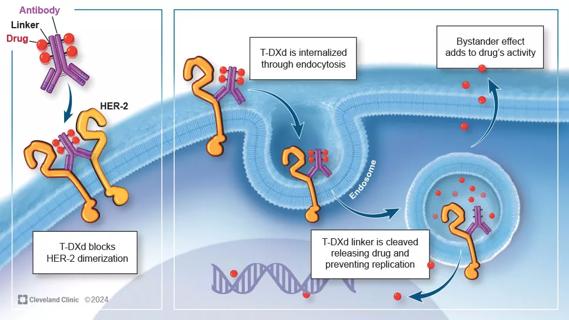
Key learnings from DESTINY trials

Overall survival in patients treated since 2008 is nearly 20% higher than in earlier patients