Investigators identify mutations that disrupt orderly processes
By Byron Lee, MD, PhD
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
Although urothelial carcinoma of the bladder is the fifth most common cancer in the United States, few major therapeutic advancements for this disease have been made over the last 20 years. The recent introduction of immunotherapy to the armamentarium of treatments for locally advanced and metastatic bladder cancer has generated much deserved excitement. As we begin to understand the detailed molecular data generated by The Cancer Genome Atlas study and other genome-wide analyses of both muscle-invasive and non-muscle-invasive disease, the potential for developing novel regimens for all stages of bladder cancer may be realized in the near future.
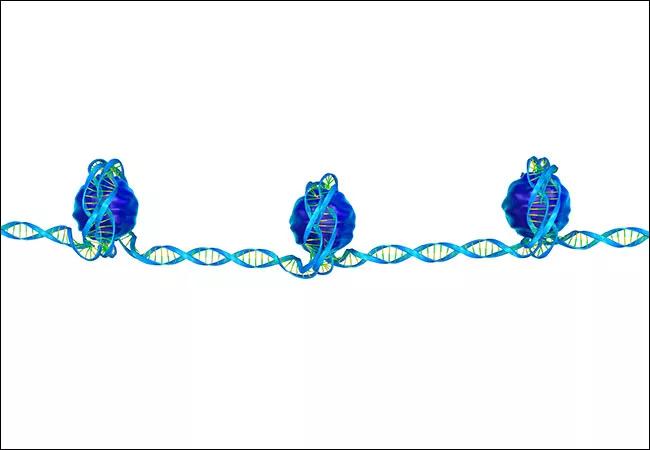
One of the most striking findings from these studies is that the majority of bladder cancers harbor a chromatin modifier gene mutation. Chromatin refers to the macromolecular complex formed when DNA is wrapped around histones. This DNA-histone interaction is critical in determining accessibility of specific DNA elements to DNA-binding proteins such as transcription factors, co-activators, co-repressors, etc. Chromatin modifiers alter the DNA-histone interface by post-translational modification of histone tails or shifting the position of histones along the DNA strand. Thus, chromatin modifiers play a central role in the regulation of proper gene expression, initiation of DNA replication, and recombination.
During differentiation, stem cells transition through distinct chromatin states that ultimately lead to repression of pluripotency genes and activation of lineage-specific genes. Chromatin modifier gene mutations disrupt this orderly process and can lead to malignancy. In bladder cancer, mutations were seen in a wide variety of chromatin modifiers, including histone methyltransferases, histone demethylases, histone acetyltransferases, and ATP-dependent nucleosome remodeling complexes. Moreover, mutations were identified in both non-muscle invasive and muscle-invasive disease, which suggests that they occur early in the disease process and can heavily influence cancer biology.
Advertisement
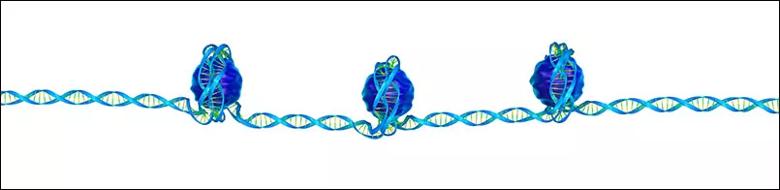
Chromatin, often described as “beads on a string,” is the macromolecular complex formed when DNA is wrapped around histones.
The functional consequences of chromatin modifier mutations in bladder cancer have not yet been elucidated. We and others are investigating how altered gene expression patterns consequent to these mutations can promote bladder cancer initiation and progression. Recently, the array of available epigenetic drugs, which inhibit chromatin modifiers, readers, or DNA methyltransferases has grown increasingly sophisticated, and these compounds will be important tools in characterizing the bladder cancer epigenome.
Some epigenetic drugs such as histone deacetylase inhibitors and DNA methyltransferase inhibitors are already FDA-approved for several hematologic malignancies. With respect to bladder cancer, Cleveland Clinic was one of 14 centers participating in an open-label, single-arm Phase 2 clinical trial (NCT02236195) examining the response of tumors deficient in the histone acetyltransferases CREBBP or EP300 to mocetinostat, a histone deacetylase inhibitor. Patient accrual has been completed, and the results are eagerly anticipated. Of particular importance will be examining how gene dysregulation due to specific chromatin modifier mutations interacts with known driver mutations in bladder cancer such as those in the RTK-RAS-RAF and PI3K-AKT-MTOR pathways.
In addition, data from other malignancies demonstrate that altered chromatin states can mediate acquired drug resistance to targeted therapies, and we need to understand how chromatin modifier mutations can potentially contribute to this mechanism. Epigenetic drugs have also been shown to potentiate response to immunotherapy, but the effects of chromatin modifier mutations in this setting will need to be ascertained.
Advertisement
Elucidating the biologic role of chromatin modifier mutations in bladder carcinogenesis will be critical in furthering our understanding of this disease. Detailed analysis of the complex interplay between epigenetic and genetic alterations will generate new targets for the design of rational therapeutic combinations and potentially improve the ability to predict and prevent drug resistance.
In the meantime, our bladder cancer program participates in cooperative oncology groups and offers numerous clinical trials.
Dr. Lee is a surgeon/scientist with a primary appointment in the Glickman Urological and Kidney Institute Department of Urology and a secondary appointment in the Lerner Research Institute Department of Cellular and Molecular Medicine. His research focuses on understanding how genomic and epigenetic alterations contribute to the development of bladder cancer, its progression and its response to therapy.
Advertisement
Advertisement

First-of-its-kind research investigates the viability of standard screening to reduce the burden of late-stage cancer diagnoses

Global R&D efforts expanding first-line and relapse therapy options for patients

Study demonstrates ability to reduce patients’ reliance on phlebotomies to stabilize hematocrit levels

A case study on the value of access to novel therapies through clinical trials

Findings highlight an association between obesity and an increased incidence of moderate-severe disease

Cleveland Clinic Cancer Institute takes multi-faceted approach to increasing clinical trial access 23456

Key learnings from DESTINY trials

Overall survival in patients treated since 2008 is nearly 20% higher than in earlier patients