Growing evidence of advantages over other radiosurgery approaches
By Lilyana Angelov, MD; Samuel Chao, MD; and Gene Barnett, MD, MBA
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
Two-staged stereotactic radiosurgery has been shown to be a feasible, safe and effective modality for treating large brain metastases in the largest published series of metastases managed with this approach to date. These results, reported recently by our group in the Journal of Neurosurgery,1 raise the prospect of enhanced local tumor control with decreased radiation-related morbidity in the setting of large brain metastases.
Effective control of large brain metastases (≥ 2 cm maximum diameter) with stereotactic radiosurgery (SRS) is a challenge, yielding local control rates of only 37 to 62 percent with an elevated risk of treatment-associated toxicity compared with smaller brain metastases. In recent years, two centers in Japan began reporting results with a novel strategy for treating large brain metastases known as staged stereotactic radiosurgery (SSRS).2-4 The approach involves delivery of SRS in two or more discrete treatment sessions rather than the traditional single session. The aim is to enable an increased overall dose to improve local tumor control while administering smaller individual doses in an effort to reduce toxicity.
In 2012, Cleveland Clinic’s Rose Ella Burkhardt Brain Tumor and Neuro-Oncology Center became, to our knowledge, the first center outside Japan to offer two-staged SRS (2-SSRS). Our new paper1 reports our experience with this approach from June 2012 through January 2016.
We retrospectively analyzed all Cleveland Clinic patients during this period who underwent planned 2-SSRS for brain metastases ≥ 2 cm in maximum diameter secondary to systemic cancer. Patients were selected for planned 2-SSRS if they were not surgical candidates or per surgeon and patient preference. The Gamma Knife® Perfexion system was used to deliver a total of 24 to 33 Gy (median, 30) across the two treatment sessions, resulting in a total biologic equivalent prescription dose of roughly 44 to 73 Gy (median, 62.5) if delivered in a single treatment session. The second SSRS session was typically scheduled approximately one month after the first (median interval, 34 days).
Advertisement
Our objective was to volumetrically assess the response of local brain metastases to the 2-SSRS strategy in terms of local control rates, treatment-related toxicity and impact on overall survival.
Fifty-four patients received 2-SSRS during the study period, with a total of 63 treated brain metastases among them: 46 patients (85 percent) had one metastasis, seven (13 percent) had two and one (2 percent) had three. Patients with more than one metastasis had them treated concurrently. Median patient age was 63 years (range, 23-83).
The main outcome findings were as follows:
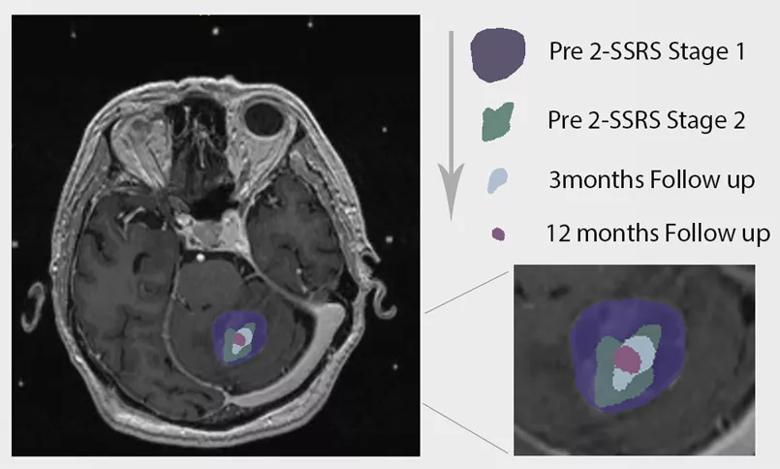
Figure 1. Tumor size over time in one of the patients from our study (image courtesy of neurosurgery resident Ghaith Habboub, MD).
Our findings build on the initial results from Japan to support 2-SSRS as a feasible, safe and effective modality that yields excellent local control and similar or better overall survival and toxicity relative to many series (reviewed in our paper1) in which large brain metastases were treated with single-session SRS or fractionated stereotactic radiosurgery (FSRS). We also showed that multiple large brain metastases can be treated concurrently with 2-SSRS and that this strategy can be effective in treating large metastases arising from traditionally radiotherapy-resistant pathology.
Advertisement
These findings suggest that a prognostic model may well be in order to stratify patients with large brain metastases into favorable and unfavorable 2-SSRS response groups based on Karnofsky Performance Status, global intracranial disease and response of the tumor to initial SSRS treatment. Such a model could be a helpful guide to clinical decision-making.
At the same time, larger prospective trials are warranted to confirm these retrospective results, assess durability and directly compare 2-SSRS to alternative approaches for large brain metastases. Nevertheless, 2-SSRS appears to offer a number of advantages over other radiosurgery strategies in the setting of large brain metastases, including:
Advertisement
Apart from these broader potential advantages, 2-SSRS appears particularly well suited to several specific applications. These include treating tumors in eloquent brain or near critical structures where radiotoxicity is especially concerning, enabling deferral of whole-brain radiation therapy (WBRT) in patients who aren’t surgical candidates, and use in patients with limited options who have already had WBRT and are not surgical candidates. We look forward to helping further define the role of this promising new approach to stereotactic radiosurgery.
Dr. Angelov (angelol@ccf.org) is a neurosurgeon in the Rose Ella Burkhardt Brain Tumor and Neuro-Oncology Center in Cleveland Clinic’s Neurological Institute.
Dr. Chao (chaos@ccf.org) is a radiation oncologist in the Rose Ella Burkhardt Brain Tumor and Neuro-Oncology Center.
Advertisement
Dr. Barnett (barnetg@ccf.org) is a neurosurgeon and Director of the Rose Ella Burkhardt Brain Tumor and Neuro-Oncology Center.
Advertisement

First-of-its-kind research investigates the viability of standard screening to reduce the burden of late-stage cancer diagnoses

Global R&D efforts expanding first-line and relapse therapy options for patients

Study demonstrates ability to reduce patients’ reliance on phlebotomies to stabilize hematocrit levels

A case study on the value of access to novel therapies through clinical trials

Findings highlight an association between obesity and an increased incidence of moderate-severe disease

Cleveland Clinic Cancer Institute takes multi-faceted approach to increasing clinical trial access 23456

Key learnings from DESTINY trials

Overall survival in patients treated since 2008 is nearly 20% higher than in earlier patients