Novel agents restore T-cell activity, produce durable response

Immune checkpoint blockade (ICB) research is one of the most exciting recent developments in immunotherapy for cancer, and Cleveland Clinic researchers are at the center of it. The use of nivolumab, alone or in combination with other agents, has shown significant promise in early trials of patients with metastatic renal cell carcinoma (mRCC), as well as cancers of the lung and skin.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
Brian I. Rini, MD, FACP, of Cleveland Clinic’s Taussig Cancer Institute, is one of the lead investigators in international studies examining the efficacy of immune checkpoint inhibitors against mRCC.
“Taussig Cancer Institute has taken a leadership role in the development of novel immunotherapies for cancer,” says Dr. Rini. “Building on a long history of clinical and laboratory excellence in immunology and immunotherapy, we are poised to develop the next generation of immunotherapeutics to extend the lives of cancer patients.”
ICB’s purpose is to temporarily inhibit modulating mechanisms in the patient’s immune system and to bring the full force of the immune system to bear against cancer cells. In a healthy person, these modulating systems protect bodily tissue from the actions of immune factors. The challenge for oncologists has been that malignant tissue is able to exploit these mechanisms and to become less susceptible to the disease-fighting abilities of activated T-cells and other immune components, including CTLA-4, PD-1, LAG-3 and TIM-3.
Restoring Immune Activity with Nivolumab
Dr. Rini and his colleagues have completed some trials – and are conducting others – involving nivolumab, an antibody that inhibits expression of the programmed death-1 (PD-1) receptor and its principal ligand, PD-L1. PD-L1 expression has been clinically associated with poor prognosis in mRCC patients.
Nivolumab is a human IgG4 PD-1 immune checkpoint inhibitor. It has been shown to restore T-cell immune activity. Patients with mRCC treated with the agent have shown positive objective responses in a phase 1 trial (N = 296) whose results were published in the New England Journal of Medicine.
Advertisement
This group found that the objective responses to nivolumab were durable in patients with advanced non-small-cell lung cancer, melanoma and RCC. At one-year follow-up, 20 of 31 patients exhibited objective responses that lasted one year or longer. There was also a strong correlation between PD-1/PD-L1 expression and response to immune blockade. Of the 17 patients with PD-L1-negative tumors, none had an objective response to ICB with nivolumab. In contrast, 9 of 25 patients with PD-L1-positive tumors had an objective response.
The researchers concluded that “anti-PD-1 antibody produced objective responses in approximately 1 in 4 to 1 in 5 patients with non-small-cell lung cancer, melanoma or renal cell cancer.”
Other ICB Study Results
Several other promising study results have been presented as abstracts at recent annual meetings of the American Society of Clinical Oncology (ASCO). Cleveland Clinic conducted some of this research.
In general, the results have revealed high objective response rates and tolerable side-effect profiles when compared with treatment with vascular endothelial growth factor (VEGF) inhibitors (bevacizumab, sunitinib and pazopanib) and with mammalian target of rapamycin (mTOR) inhibitors (everolimus and temsirolimus).
In contrast, one study presented at ASCO found that up to 31 percent of patients with heavily pretreated mRCC were responsive to PD-1/PD-L1 blockade. The responses have been durable and associated with relatively little grade-3 toxicity.
In a phase II dose-ranging trial presented at ASCO, the researchers, including Dr. Rini, observed significant anti-tumor activity following treatment with nivolumab. In a group of patients (N = 168) with pretreated mRCC, all three dosages (0.3 mg/kg, 2 mg/kg and 10 mg/kg) were associated with objective responses of long duration. The median duration of objective response was 18.2 months for the 0.3 mg/kg group, which was not reached in the groups receiving the higher dosages. Rates of grade 3-4 treatment-related adverse events were ≤ 17 percent in all three groups. There were no instances of grade 3-4 pneumonitis.
Advertisement
A phase I trial of nivolumab in combination with ipilimumab, also presented at ASCO by a group that included Dr. Rini, showed similarly promising results. Patients were randomly assigned to one of two arms in this trial: nivolumab 3 mg/kg plus ipilimumab 1 mg/kg (arm N3+I1; N = 6) or nivolumab 1 mg/kg plus ipilimumab 3 mg/kg (arm N1+I3; N = 9). Durations of response were 4.1+ to 22.1+ weeks in the N3+I1 arm and 6.1+ to 18.3+ weeks in the N1+I3 arm. Stable disease was observed in 39 percent of patients in N3+I1 and 33 percent of patients in N1+I3. Responses to treatment occurred by week 6 (first tumor assessment) in 67 percent of patients in both groups.
Cleveland Clinic researchers are also involved in international studies of two other checkpoint inhibitors: pembrolizumab and MPDL3280A. Study of these two compounds is ongoing, but early results suggest they have the potential for clinical efficacy.
Dr. Rini intends to continue with this promising ICB research, investigating ways to further reduce adverse treatment-related side effects, identify optimal dosages and dosing schedules, and discover other chemotherapeutic agents that act synergistically with immune checkpoint inhibitors.
Dr. Rini is a staff member of Cleveland Clinic’s Department of Hematology and Medical Oncology and an Associate Professor of Medicine at the Cleveland Clinic Lerner College of Medicine.
Advertisement
Advertisement

First-of-its-kind research investigates the viability of standard screening to reduce the burden of late-stage cancer diagnoses

Global R&D efforts expanding first-line and relapse therapy options for patients
Study demonstrates ability to reduce patients’ reliance on phlebotomies to stabilize hematocrit levels

A case study on the value of access to novel therapies through clinical trials

Findings highlight an association between obesity and an increased incidence of moderate-severe disease
Cleveland Clinic Cancer Institute takes multi-faceted approach to increasing clinical trial access 23456
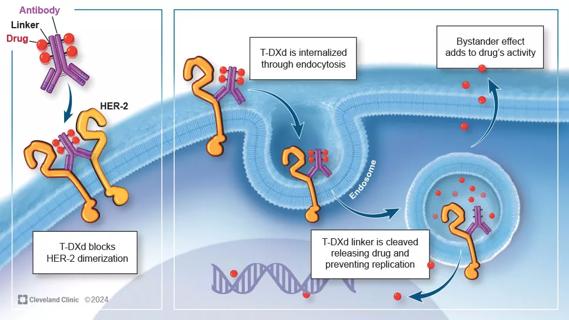
Key learnings from DESTINY trials

Overall survival in patients treated since 2008 is nearly 20% higher than in earlier patients