Novel design hopes to overcome current limitations

Cleveland Clinic Children’s is one of a few selected centers in the U.S. participating in clinical trials of a novel device for transcatheter closure of secundum atrial septal defects (ASD). It is hoped the new device, known as the Gore CARDIOFORM ASD Occluder, will overcome the rare, yet significant, complication that can occur from the only device currently available to close large defects.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
“The investigative device is more flexible and conforms better to intracardiac structures. It is, therefore, less likely to cause injury to the heart,” says Lourdes Prieto, MD, Director of the Pediatric Catheterization Laboratory at Cleveland Clinic.
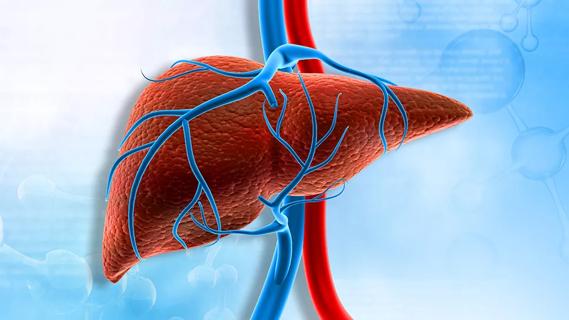
A secundum ASD is a hole in the wall between the upper chambers of the heart that occurs when the atrial septum fails to close after birth. The hole allows oxygenated blood from the left atrium to pass through the septum and mix with poorly oxygenated blood in the right atrium. Over time, this can lead to right heart failure, arrhythmias and pulmonary hypertension. In the worst-case scenario, the patient may require a heart-lung transplant.
About 85 percent of secundum ASDs can be closed without surgery in a catheter-based procedure. Two FDA-approved ASD occluders are available for this procedure: The Amplatzer Septal Occluder, and a different Gore model, the Fore CARDIOFORM Septal Occluder. Both devices have been proven to be safe and effective when compared with surgery to close the ASD. However, the devices have limitations based on the size of the defect and the amount of available tissue around the hole on which the device is anchored.
The available Gore can only be used in defects up to a certain size. Larger defects require the Amplatzer occluder be used. About 1 in 300 patients who receive the Amplatzer device suffer erosion of heart tissue, resulting in pericardial effusion. The complication is not predictable, and the time frame is variable.
Advertisement
“It most commonly appears in the first few weeks to months after device implantation, but it can occur several months — or even years — later,” says Dr. Prieto. “There is no way to monitor patients for this potential complication; we advise them to seek immediate care if they experience chest pain, shortness of breath or dizziness.”
The Gore CARDIOFORM ASD Occluder is actively being investigated in the ASSURED IDE trial and is not approved for commercial use in the U.S. It is designed to cover large ASDs. It is formed from two disks covered with a Goretex membrane stretched over a petal-like wire frame. One disk is deployed on each side of the defect. Unlike the older Gore model, which has a thin waist joining the two disks, the new occluder fills the defect with membrane.
“When the device is deployed, it does not apply excessive pressure on surrounding tissue, which could lead to cardiac injury,” says Dr. Prieto.
ASD is rarely symptomatic in childhood. Rather, it is usually discovered when a murmur prompts a pediatrician to refer the patient to a cardiologist. The diagnosis is confirmed with an echocardiogram.
“We recommend closing the defect around age 4 or 5. There is nothing to be gained by waiting,” says Dr. Prieto.
When an ASD is diagnosed in adulthood, the hole should be closed as quickly as possible, since the risk of irreversible complications increases by age starting in young adulthood.
Enrollment in the ASSURED IDE trial has stopped, as the target number of participants has been reached. Follow-up of each patient will be conducted at six months post implant.
Advertisement
Cleveland Clinic Children’s plans to participate in the Continued Access phase of ASSURED, which is expected to begin later this year.
“We hope this ASD occluder will decrease the number of patients whose device is associated with erosion,” says Dr. Prieto.
Advertisement
Advertisement

Systemic change needed to improve health outcomes for parents and children, say researchers
Rare genetic variant protected siblings against seizures and severe hypoglycemia

Movie has more positive impact than expected, says Head of Adolescent Medicine

Genetic changes are similar between some vascular anomalies and cancers
Expert panel advises a two-tier structure for surgical centers
Our new head of pediatric general and thoracic surgery shares his passion and vision
Basic understanding of condition and treatment is lacking