Novel autoantigens are identified, precise roles unclear
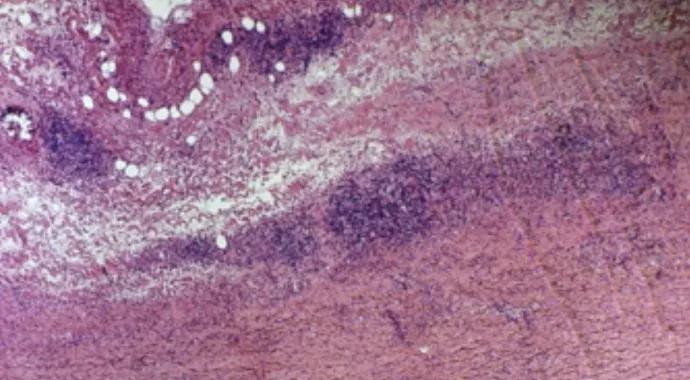
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/9cabcfac-f9ca-469e-89c0-95e77a68e410/15-RHE-539-Hoffman-Hero-Image-690x380pxl_jpg)
15-RHE-539-Hoffman-Hero-Image-690x380pxl
By Gary S. Hoffman, MD, MS, MACR
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
Large-vessel vasculitides (LVV) are a group of autoimmune diseases characterized by injury to large vessels that may include the aorta and its branch vessels (Figure 1). Disease etiology is unknown. As with all presumed autoimmune diseases, investigators have searched for those antigens that may be driving the immune response. If one were fortunate enough to find the culprit antigen, the next question would be whether it was a native antigen to which immune tolerance was lost or a modified antigen to which a seemingly appropriate immune response was mounted. Understanding these pathways would provide unique opportunities for treatment.
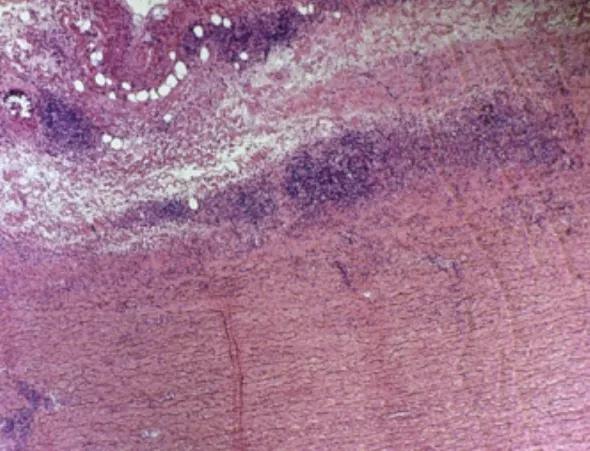
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/6e3ea7ec-2528-4d31-afee-d16433f29c8e/15-RHE-539-Hoffman-Inset-Image-Fig-1-590pxl-width_jpg)
Figure 1. Aortic arch specimen from a patient with an enlarging aneurysm due to a necrotizing inflammatory process (10× magnification, H&E stain).
Increasing specialization and sophistication in medical science require that pursuing answers to pathogenesis questions is best accomplished by multidisciplinary teams. Cleveland Clinic’s LVV team includes rheumatologists, cardiothoracic surgeons, biochemists, immunologists, geneticists and an ophthalmologist.
Using thoracic aorta surgical specimens, we sought to identify antigen targets within affected vessels in patients with LVV, including giant cell arteritis (GCA), Takayasu arteritis and isolated focal aortitis. In parallel studies, we used aortic and temporal artery specimens to determine whether infectious agents play a role in GCA. Each study is a “first” in addressing antigen discovery because it utilizes biochemical and microbiome techniques in snap-frozen sterile specimens.
Advertisement
In the aorta studies, we found that patients with LVV produce antibodies to regulatory 14-3-3 proteins, whereas patients with noninflammatory aorta matrix disorders rarely do so. Anti-14-3-3 antibody was demonstrated in all three forms of LVV. Antibodies to 14-3-3 were usually not found in sera of normal and autoimmune disease controls (Figure 2).
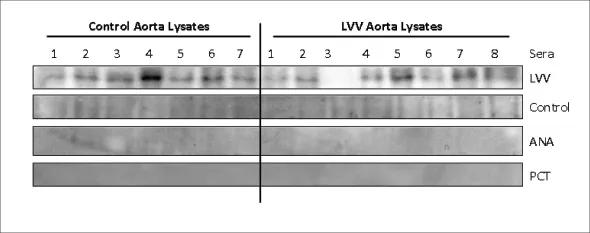
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/20154225-0fd4-4fde-993c-85f4711e4807/15-RHE-539-Hoffman-Inset-Image-Fig-2-590pxl-width_jpg)
Figure 2. LVV serum shows reactivity to ~30-kd protein. Aortic lysates from controls and LVV tissues were resolved on 10 percent SDS-PAGE. After transfer, PVDF membranes were first blotted with control serum and later stripped for reprobing with LVV serum. Representative gels are shown for each serum blot. Two nonvasculitic serum controls (PCT- and ANA-positive sera) also were screened for reactivity and were negative. Subsequent mass spectroscopic analysis of the 30-kd region on protein gel identified 14-3-3 to be the most abundant protein in this region, particularly in LVV samples. (From the laboratory of Ritu Chakravarti, PhD
14-3-3 proteins appear to be novel autoantigens in LVV-associated aortic aneurysms. The precise role these antibodies and 14-3-3 proteins play in LVV pathogenesis will be further studied to clarify the following:
Aortic and temporal artery specimens also were used to explore whether infectious agents resided in LVV tissue. Cleveland Clinic’s Genomic Medicine Institute/microbiome team (Pauline Funchain, MD, and Charis Eng, MD, PhD ‒ see Acknowledgments box at end) discovered that all thoracic aortic aneurysms and temporal arteries hosted bacterial communities of varying abundance.
Advertisement
LVV-associated aorta microbiome clusters differed by disease type, with the clearest separation seen between focal isolated aortitis and Takayasu samples. The non-GCA control temporal artery biopsy microbiomes clustered tightly together, showing high degrees of taxonomic relatedness, while GCA microbiomes did not cluster and were clearly separated from controls.
We can state with a high degree of certainty that thoracic aortic aneurysms and temporal arteries are not sterile and that specimens from patients with different disease associations host distinct microbial communities.
Dr. Hoffman was a staff physician in the Department of Rheumatic and Immunologic Diseases and Professor of Medicine, Cleveland Clinic Lerner College of Medicine.
The author is privileged to summarize this work on behalf of the following Cleveland Clinic colleagues:
Advertisement
Advertisement
Lorem ipsum dolor sit amet. Ut laudantium quasi et aliquam magni qui adipisci sequi et voluptatibus blanditiis et consectetur atque eos nihil dolorem qui culpa nobis. In accusantium voluptatem est modi rerum ut aperiam neque. Hic quos nisi 33 rerum laboriosam cum fuga voluptates. Sed expedita ratione sit nostrum necessitatibus ea maxime perspiciatis sed quia sequi.
This is a subtitle for CQD Post 'Corey Test CQD Post Feb12'. This is a subtitle for CQD Post 'Corey Test CQD Post Feb12'.</end Subtitle>
Matching Secondary -> Primary Snomed Term with Curated Post. Please do not touch this post or any of its categories/snomed terms, thank you!
Lingulectomy removes infection when antibiotics fail
Researchers have developed immunoprofiles for an emerging disease with a mortality rate as high as 27%