Biofilm explains IE’s resistance to nonsurgical treatment
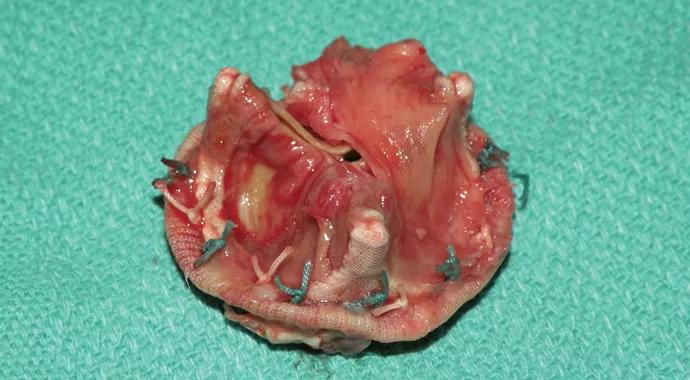
New molecular microbiology techniques are uncovering the microorganisms that evade antimicrobial treatment for infective endocarditis (IE) — and offering possible ways to combat the culprit organisms.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
IE is associated with significant morbidity and mortality. In most cases, prolonged courses of broad-spectrum antimicrobials fail to eradicate the infection, making surgical intervention the only solution.
Gosta Pettersson, MD, PhD, Vice Chairman of Cleveland Clinic’s Department of Thoracic and Cardiovascular Surgery and Section Head of Congenital Heart Surgery, led a Cleveland Clinic team that recently found evidence strongly suggesting that IE is a biofilm-associated infection, explaining its resistance to nonsurgical treatment. They have submitted their findings to the Journal of Thoracic and Cardiovascular Surgery.
The term biofilm describes a surface mode of infection in which microbes attach to a surface and grow in self-contained micro-colonies with a protective extracellular polymeric matrix — also referred to as a slime layer. Located near the surface of the biofilm are free planktonic cells that can spread to colonize surrounding structures and other organs.
The bigger problem lies deep within the biofilm structure, where so-called persister cells rebuild and develop the biofilm. The body’s immune response and antimicrobial drugs are unable to penetrate the biofilm. “In this way, the biofilm concept explains why IE management is clinically challenging,” notes Dr. Pettersson.
The National Institutes of Health reports that up to 80 percent of clinical infections involve biofilm bacterial phenotypes, particularly those associated with IE and implanted medical devices. Biofilm has been identified on cardiac pacemakers, prosthetic heart valves, vascular grafts, intravascular catheters, orthopaedic implants, sternal fixation wires and endotracheal tubes.
Advertisement
The most common biofilm-forming organisms are Staphylococcus epidermidis, Staphylococcus aureus, Streptococcus species, Pseudomonas aeruginosa and Enterobacteriaceae microorganisms.
Despite advances in medical and surgical therapies, IE is notoriously difficult to treat, even with extended use of high-dose intravenous antibiotics. IE is associated with in-hospital mortality rates of 20 percent — and five-year mortality rates double that. High-risk surgery is required in half of all IE cases to control infection.
Dr. Pettersson and his colleagues note that biofilm formation is a new explanation for the difficulty encountered in treating these infections — and, importantly, that surgery disrupts the biofilm and exposes these microbes to antibiotics.
Chronic infection with recurrent aggravation of symptoms is a hallmark of the biofilm mode of infection. Antimicrobial therapy kills planktonic bacterial cells in the blood, leading to temporary improvement. But a relapse of systemic symptoms occurs as more bacterial cells are released from the biofilm at some point following antibiotic treatment.
New antimicrobial therapies are being developed to attack biofilm, and new pharmacologic strategies are targeting phases of the biofilm growth model. But today there are no therapies strong enough to penetrate the biofilm to reliably cure prosthetic valve IE without surgery. Dr. Pettersson says additional research is needed to better characterize the microbiologic aspects of IE and to develop effective anti-biofilm therapies.
Advertisement
Advertisement

A sampling of outcome and volume data from our Heart & Vascular Institute
Concomitant AF ablation and LAA occlusion strongly endorsed during elective heart surgery
Large retrospective study supports its addition to BAV repair toolbox at expert centers
Young age, solid tumor, high uptake on PET and KRAS mutation signal risk, suggest need for lobectomy
Surprise findings argue for caution about testosterone use in men at risk for fracture
Residual AR related to severe preoperative AR increases risk of progression, need for reoperation

Findings support emphasis on markers of frailty related to, but not dependent on, age
Provides option for patients previously deemed anatomically unsuitable