Cementless designs facilitate improved options
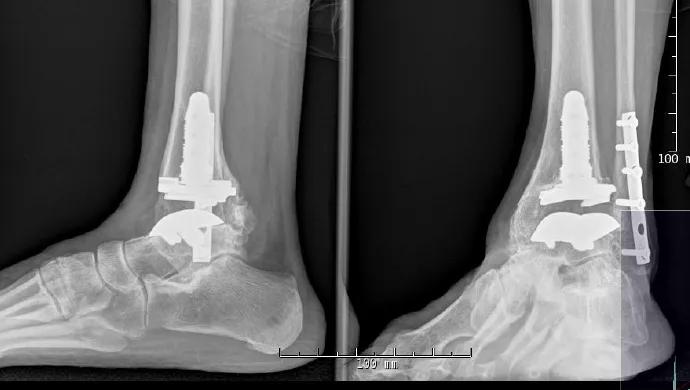
By James J. Sferra, MD, and Eva Umoh Asomugha, MD
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
Ankle fusion or total ankle replacement (TAR)? It’s one of the biggest dilemmas faced by patients with end-stage ankle arthritis and their orthopaedic surgeons. Ankle arthrodesis has been the gold standard, but development of both radiographic and clinically relevant degenerative disease in the adjacent joint at long-term follow-up has made TAR an attractive option in appropriate patients. For patients requiring bilateral ankle fusions or those with end-stage ankle arthritis and previous hindfoot or midfoot fusions, studies demonstrate that ankle fusions have unfavorable effects on gait and functional capability.
First-generation ankle replacements, which required cemented fixation and significant bone resection, were hindered by high complication and failure rates. Renewed interest in more anatomically based, cementless designs spurred development of second- and now third-generation implants, which have demonstrated improved results at short- and medium-term follow-up. The hallmarks of third-generation implants are cementless fixation, with minimal bone resection as a result, and a polyethylene articulation. Outcomes, in terms of pain scores, range of motion and patient-rated functional capacity, have been promising with newer-generation designs.1
Complications with TAR are prevalent and are often related to patient-related risk factors or technical errors. Reported complications include infection, delayed wound healing, stress fractures (of the malleolus or distal tibia), osteolysis and implant migration or subsidence. Proper patient selection is critical to increasing the longevity of the prosthesis and minimizing these complications. TAR can be used in patients with idiopathic, post-traumatic or inflammatory arthritides. Ideal candidates are middle-aged or elderly nonlaborers who have adequate bone stock, little to no deformity, and a normal foot and ankle neurovascular exam.
Advertisement
Of the several FDA-approved TAR implant systems, Cleveland Clinic currently uses the following three: • The Scandinavian Total Ankle Replacement System (STAR™ Ankle, Small Bone Innovations) is the only FDA-approved cementless, three-piece, mobile-bearing implant. One of the proposed benefits of this system is the limited bone resection needed for implantation, which should make future conversion or revision more feasible if necessary. Five- and 10-year implant survival rates as high as 90 percent and 80 percent, respectively, have been reported.2 The mobile-bearing system increases the planes of motion, allowing some varus and valgus tilt in the mortise during ambulation. • The Inbone® Total Ankle System (Wright Medical Technology) is a modular, fixed-bearing, two-component system with intramedullary tibial and talar components. The modularity allows different-sized tibial and talar components to be used to match the patient’s native anatomy. Preoperative CT scans can be used to fabricate preoperative navigation alignment guides to serve as patient-specific instruments to assist in intraoperative positioning of the TAR components. These guides also allow the surgeon to avoid use of the Inbone leg holder intraoperatively, which can be very helpful in the OR. This system works well for primary cases as well as revisions. • The Salto Talaris™ Total Ankle Prosthesis (Tornier) has a design and instrumentation founded on the Salto mobilebearing ankle prosthesis, which has been in clinical use in Europe since 1997. Studies demonstrate greater than 90 percent implant survival at midterm follow-up.3 A key principle of this system is that the mobile-bearing concept has been moved from the final implant into the stage of trialing instrumentation. Specifically, the trial tibial base is allowed to rotate into proper position by moving the ankle through an arc of motion, thus allowing the prosthesis to self-align. The tibial keel preparation is then completed, essentially locking the components into their appropriate positions. The Trabecular Metal™ Total Ankle (Zimmer) was recently approved in the U.S. but has not yet been implanted at our institution. It is a semiconstrained device with three implant components designed to be implanted via a lateral malleolus osteotomy.
Advertisement
At Cleveland Clinic, we consider many factors when choosing whether to perform ankle fusion or TAR ‒ and then, if TAR is pursued, which implant to use. The patient’s weight, age, angular deformity, soft tissues and activity level are all considered. The aim is to match these patient characteristics to the implant system in order to minimize soft tissue dissection and bone resection and preserve the ankle’s natural anatomy and kinematics.
1. Stengel D, Bauwens K, Ekkernkamp A, Cramer J. Efficacy of total ankle replacement with meniscal-bearing devices: a systematic review and meta-analysis. Arch Orthop Trauma Surg. 2005;125(2):109-119.
2. Wood PL, Prem H, Sutton C. Total ankle replacement: medium-term results in 200 Scandinavian Total Ankle Replacements. J Bone Joint Surg Br. 2008;90(5):605-609.
3. Schweitzer KM, Adams SB, Viens NA, et al. Early prospective clinical results of a modern fixed-bearing total ankle arthroplasty. J Bone Joint Surg Am. 2013;95(11):1002-1111.
Dr. Sferra specializes in foot and ankle reconstructive surgery in the Department of Orthopaedic Surgery. He can be reached at 216.445.8507 or sferraj@ccf.org.
Dr. Asomugha is a PGY-5 resident in the Department of Orthopaedic Surgery. She can be reached at asomuge@ccf.org.
Advertisement
Advertisement
Biologic approaches, growing implants and more
Study reports zero infections in nearly 300 patients
How to diagnose and treat crystalline arthropathy after knee replacement
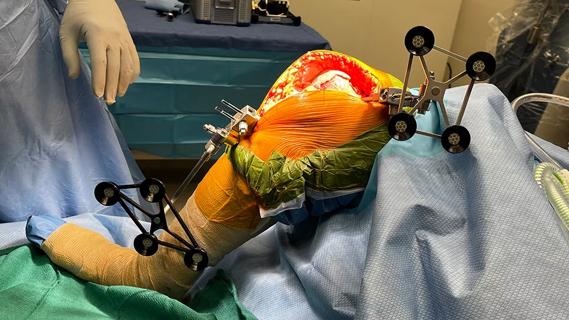
Study finds that fracture and infection are rare
Center will coordinate, interpret and archive imaging data for all multicenter trials conducted by the foundation’s Osteoarthritis Clinical Trial Network

Reduced narcotic use is the latest on the list of robotic surgery advantages

Cleveland Clinic specialists offer annual refresher on upper extremity fundamentals
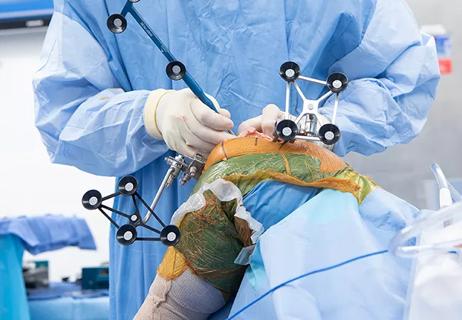
Cleveland Clinic orthopaedic surgeons share their best tips, most challenging cases and biggest misperceptions