FDA approvals for valve-in-valve TAVR follow clearance of 3rd-generation valve
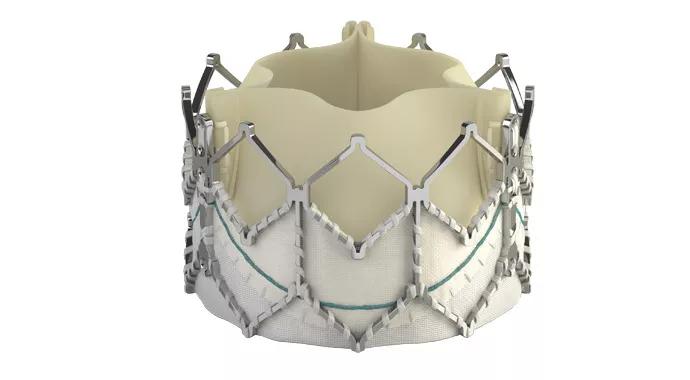
Support for transcatheter “valve-in-valve” repair as the default option for failing aortic bioprostheses in high-risk surgical patients expanded earlier this month with the FDA’s approval of the Sapien XT transcatheter valve for this new indication.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
The approval was based on one-year outcomes data from the PARTNER II Valve-in-Valve Study, which were presented at the recent Transcatheter Cardiovascular Therapeutics meeting (TCT) in San Francisco. The decision makes the Sapien XT, from Edwards Lifesciences, the second device approved in the U.S. for aortic valve-in-valve transcatheter aortic valve replacement (TAVR). Medtronic’s CoreValve System was cleared for the indication earlier this year.
“Although there are currently no randomized data comparing valve-in-valve TAVR against redo surgery, it’s definitely standard of care at this stage for high-risk surgical patients,” said Samir Kapadia, MD, at a TCT press conference where the PARTNER II Valve-in-Valve results were presented. Dr. Kapadia is Director of Cleveland Clinic’s Sones Cardiac Catheterization Lab and an investigator in studies that supported the new indication for the Sapien XT.
The PARTNER II Valve-in-Valve Study was a nonrandomized cohort analysis in which 97 patients received the Sapien XT valve from June 2012 through April 2013 and another 100 received it during an extended registry period from May to December 2013.
Key one-year outcomes data reported at TCT were as follows:
Dr. Kapadia says that while these data are impressive and another device option is welcome for valve-in-valve replacement for patients with failed valves deemed too high-risk for surgical valve replacement, selection of appropriate patients for the procedure is key.
Advertisement
“The most important thing to understand from this study is the exclusions,” he notes. “Remember that this registry went down to 21-mm surgical valves, so we can’t go below that 21-mm valve threshold.” He adds that the findings from this analysis cannot be compared directly with those supporting approval of the CoreValve device for valve-in-valve TAVR because that device can be used in smaller valve diameters in high-risk patients.
This expansion of the Sapien XT’s indications follows on the heels of the June FDA approval of the Sapien 3 valve for use in TAVR for patients with aortic stenosis who are unable to undergo surgical aortic valve replacement or deemed to be at high surgical risk.
As the third generation in the Sapien line of TAVR devices first approved in 2011, the Sapien 3 incorporates a newly added outer “sealing skirt” designed to minimize paravalvular leaks.
“Previous valves could not seal properly in all patients,” explains Dr. Kapadia, “so anywhere from 12 to 16 percent of patients had moderate leakage after these valves were placed. Paravalvular leaks make the heart work harder, so it was a serious problem.”
Other design changes in the newest iteration of the device include a lower frame height and delivery profile as well as refined frame geometry to facilitate easier placement.
Approval of the Sapien 3 device was based on results among 583 patients with aortic stenosis in the PARTNER II S3 trial who were inoperable or considered high-risk for surgery. All underwent TAVR with the Sapien 3 device in a nonrandomized single-arm design with historical controls. As presented at the American College of Cardiology Scientific Session last March, key outcomes were as follows:
Advertisement
“These results were outstanding,” says Dr. Kapadia, who served as a coinvestigator in the PARTNER II S3 trial. “The mortality and stroke rates were remarkably low.”
Additionally, rates of severe and moderate paravalvular leaks were 0.1 percent and 3.7 percent — markedly lower than the 12 to 16 percent seen with prior generations of TAVR valves.
Those paravalvular leak rates are from the entire PARTNER II S3 cohort, which includes 1,076 patients at intermediate surgical risk in addition to the 583 inoperable or high-risk patients. Full one-year data for this cohort of intermediate-risk patients are expected at the American College of Cardiology meeting in spring 2016.
Dr. Kapadia notes that Cleveland Clinic has been using the Sapien 3 in the intermediate-risk population as part of this “S3 Intermediate” arm of the trial. “When these trial results are available, we’ll know whether this latest generation of the valve can be expected to be approved for intermediate-risk aortic stenosis patients as well,” he says.
Credit: Image at top of post is courtesy of Edwards Lifesciences.
Advertisement
Advertisement

A sampling of outcome and volume data from our Heart & Vascular Institute
Concomitant AF ablation and LAA occlusion strongly endorsed during elective heart surgery
Large retrospective study supports its addition to BAV repair toolbox at expert centers
Young age, solid tumor, high uptake on PET and KRAS mutation signal risk, suggest need for lobectomy
Surprise findings argue for caution about testosterone use in men at risk for fracture
Residual AR related to severe preoperative AR increases risk of progression, need for reoperation

Findings support emphasis on markers of frailty related to, but not dependent on, age
Provides option for patients previously deemed anatomically unsuitable