In agreement with international group’s recommendations

The International Hypoglycaemia Study Group has recommended that blood glucose levels lower than 3.0 mmol/L (54 mg/dL) be recorded in clinical trials of glucose-lowering therapies. Group members agreed that this level “is sufficiently low to indicate serious, clinically important hypoglycemia,” using terms that include “serious,” “major,” or “clinically significant.”
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
The recommendation was published in Diabetes Care and issued as a joint statement by the American Diabetes Association and the European Association for the Study of Diabetes.
Cleveland Clinic staff endocrinologist and director of clinical research Kevin Pantalone, DO, has stated support for the recommendation, saying, “The glucose concentration suggested by the study group to be used for reporting hypoglycemia in clinical trials appears appropriate, as it is a distinctly low level that generally does not occur under physiological conditions in individuals without diabetes.”
For details, see this article in Endocrinology Adviser.
Advertisement
Advertisement
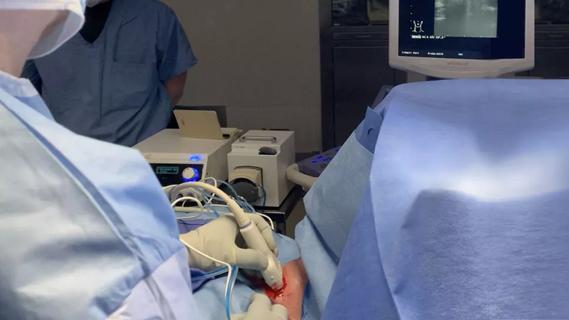
Radiofrequency ablation significantly reduces symptom severity, shrinks nodules

Maternal-fetal medicine specialists, endocrinologists and educators team up

Giving young patients a hand as they take charge of their own health

Case illustrates how easily condition can mimic preeclampsia
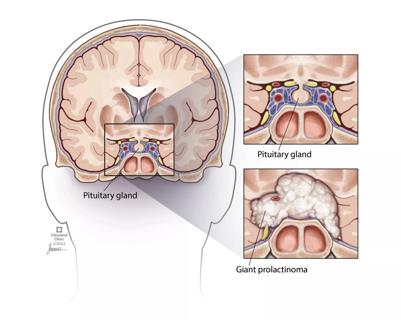
Analysis examines surgical resection of rare pituitary tumors
Screening and medication key to better outcomes
Spinal cord stimulation can help those who are optimized for success
Incidence, outcomes and management