Aims to assess ibudilast, identify imaging biomarkers
By Robert J. Fox, MD, and Ken Sakaie, PhD
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
Despite dramatic advances in the treatment of relapsing-remitting multiple sclerosis (MS), there remains no effective therapy to slow the insidious neurologic decline in progressive forms of MS. To compound the problem, there are no effective biomarkers to screen putative therapies for progressive MS.
To help fill this void, Cleveland Clinic is leading a phase 2 clinical trial with a dual objective:
If successful, the trial will provide proof-of-concept evidence supporting the efficacy of a new therapy and guide the conduct of future phase 2 trials in progressive MS.
MS typically starts as a relapsing-remitting disease, with intermittent bouts of inflammation that manifest as new lesions on conventional MRI. Over the past 20 years, more than a dozen therapies have been approved to treat relapsing-remitting MS, reducing this form of MS to an eminently treatable condition.
After 10 to 20 years, however, relapsing-remitting MS often transitions into a gradually progressive form — secondary progressive MS — in which neurologic disability accumulates little by little over time. In about 10 percent of MS cases, relapses are not seen and the disease goes directly into a progressive form, known as primary progressive MS.
None of the therapies that have proved effective in relapsing-remitting MS have demonstrated efficacy in either secondary progressive or primary progressive MS (collectively referred to as “progressive MS”). This is likely because progressive MS is a degenerative disorder that arises from the inflammatory injury of relapsing-remitting MS but is separate and independent from that inflammatory injury.
Advertisement
New lesions on MRI are commonly used as a biomarker to screen potential anti-inflammatory therapies in phase 2, proof-of-concept clinical trials in relapsing-remitting MS. Unfortunately, new lesions are uncommon in progressive MS and appear to have little relationship to the degeneration that drives progressive MS. A different biomarker is needed to screen for potential therapies in progressive MS.
Investigators from Cleveland Clinic are leading a multicenter clinical trial to address both of these problems — the lack of effective therapies and the lack of validated biomarkers for proof-of-concept studies.
The Secondary and Primary pRogressive Ibudilast NeuroNEXT Trial in Multiple Sclerosis (SPRINT-MS) is a two-year, 250-subject, randomized, placebo-controlled trial enrolling at 28 centers across the U.S. It will evaluate the safety and efficacy of ibudilast — an inhibitor of both phosphodiesterase and macrophage migration inhibitory factor (MIF) —in patients with either primary or secondary progressive MS.
The $13 million study is funded principally through the National Institutes of Health (NIH), with additional support from the National Multiple Sclerosis Society and the pharmaceutical company MediciNova. It is being conducted through the NIH-funded NeuroNEXT network, a phase 2 clinical trial network intended to accelerate development of therapies for neurologic conditions.
The trial’s primary outcome measure is whether ibudilast treatment can slow the progression of whole brain atrophy compared with placebo treatment.
Advertisement
Key secondary outcomes include cortical atrophy and brain changes on diffusion tensor imaging (DTI) and magnetization transfer ratio (MTR) imaging, all of which are thought to be sensitive metrics of neurodegeneration. Another key secondary outcome is retinal nerve fiber layer (RNFL) thinning as measured by optical coherence tomography (OCT), which is a quick and inexpensive tool for measuring MS injury in the back of the eye.
Additional outcomes include clinical disability and patient-reported outcomes.
Regardless of whether ibudilast is effective in slowing the progression of degeneration in progressive MS, SPRINT-MS also provides an opportunity to directly compare the five key outcomes:
By evaluating the longitudinal change in these measures — several of which are depicted in Figures 1 to 4 — and their correlation with disability, the study will identify the best biomarker for use in future phase 2 trials of progressive MS.
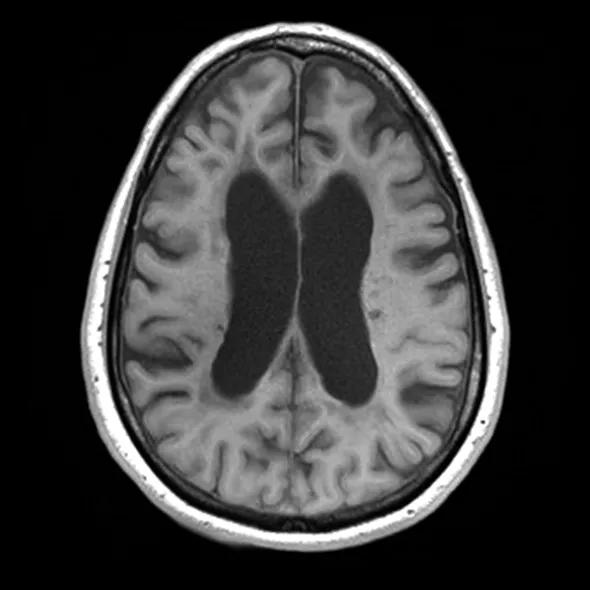
Figure 1. A conventional MRI showing severe brain atrophy, with enlarged lateral ventricles and cortical sulci.
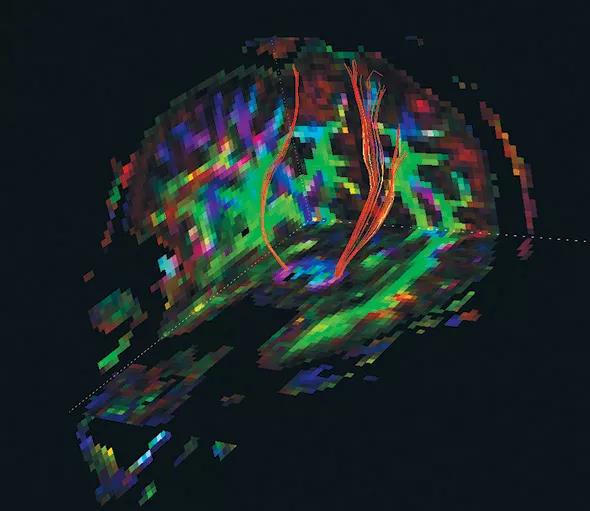
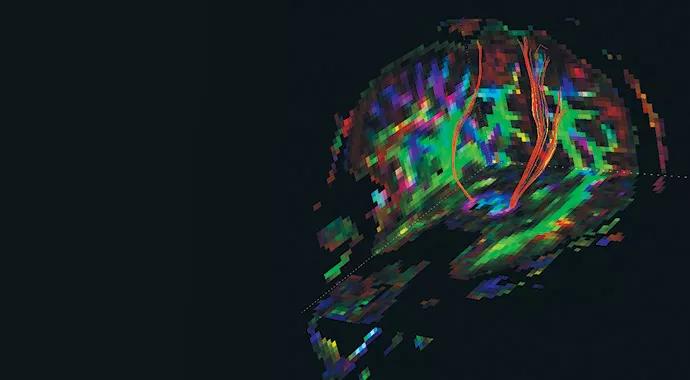
Figure 2. Rendering of corticospinal tracts from a diffusion tensor imaging (DTI) study acquired in the SPRINT-MS trial.
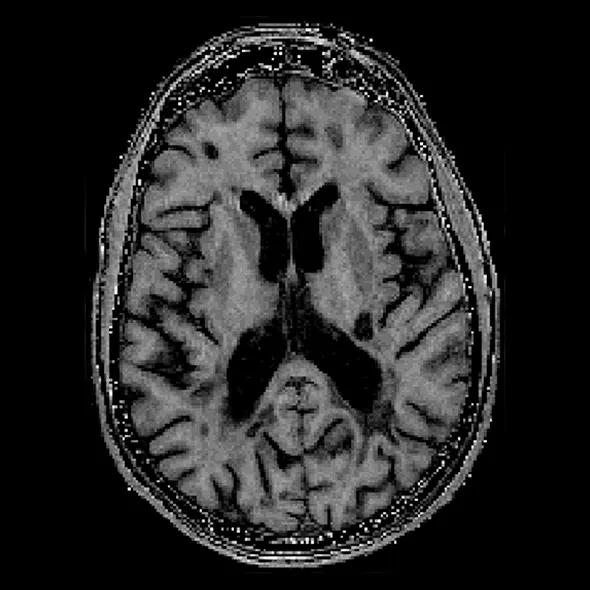
Figure 3. Magnetization transfer ratio (MTR) imaging, with MS lesions showing decreased magnetization transfer. (Image courtesy of Sridar Narayanan, PhD, McGill University.)
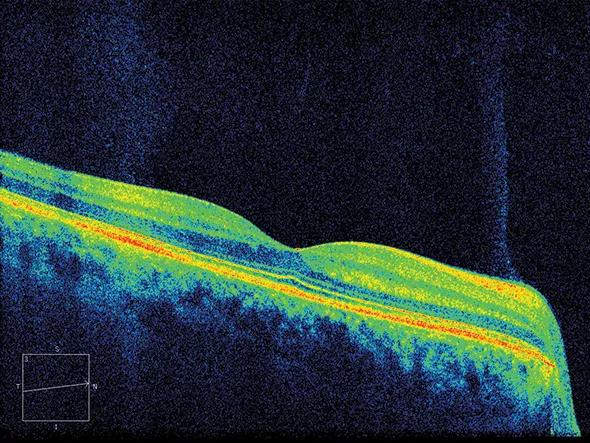
Figure 4. Optical coherence tomography (OCT) showing the retinal nerve fiber layer in the back of the eye.
Advertisement
SPRINT-MS leverages several Cleveland Clinic strengths:
Thus, the SPRINT-MS trial assimilates assets from diverse disciplines across Cleveland Clinic for a unified purpose: testing a new drug for a currently untreatable disease. And the study’s greatest significance may ultimately lie in its objective of identifying the best imaging biomarker for future progressive MS trials. In short, SPRINT-MS aims not only to catch a fish, but to teach researchers how to fish better.
Dr. Fox is a staff neurologist in the Mellen Center for Multiple Sclerosis Treatment and Research and Vice Chair for Research in the Neurological Institute.
Dr. Sakaie is an assistant staff member in the Department of Diagnostic Radiology and the Mellen Center for Multiple Sclerosis Treatment and Research.
Advertisement
Advertisement

New study advances understanding of patient-defined goals

Testing options and therapies are expanding for this poorly understood sleep disorder
Real-world claims data and tissue culture studies set the stage for randomized clinical testing
Digital subtraction angiography remains central to assessment of ‘benign’ PMSAH
Cleveland Clinic neuromuscular specialist shares insights on AI in his field and beyond

Findings challenge dogma that microglia are exclusively destructive regardless of location in brain

Neurology is especially well positioned for opportunities to enhance clinical care and medical training
New review distills insights from studies over the past decade