Findings may be due to faster atrophy progression in primary progressive MS
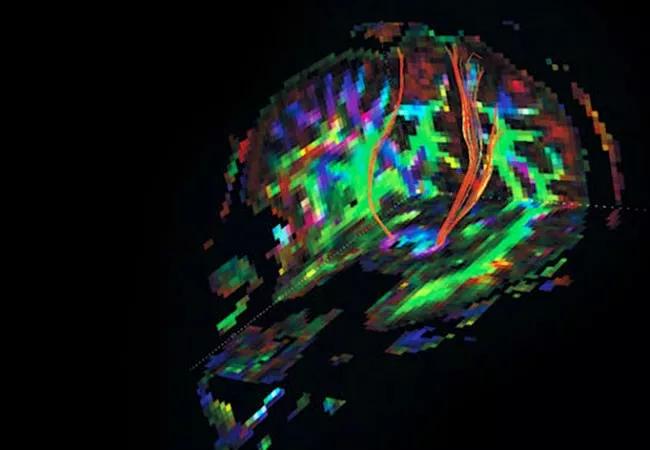
Response to the experimental oral drug ibudilast in progressive forms of multiple sclerosis (MS) appears to be driven by effects in patients with primary progressive MS (PPMS) rather than secondary progressive MS (SPMS), according to an analysis of the SPRINT-MS trial presented May 6 at the 2019 annual meeting of the American Academy of Neurology.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
“Much to our surprise, this analysis calls into question a growing sense among people in the field that PPMS and SPMS are similar forms of MS,” says senior author Robert Fox, MD, Vice Chair for Research in Cleveland Clinic’s Neurological Institute and a neurologist in its Mellen Center for Multiple Sclerosis Treatment and Research. “This dataset suggests instead that there may be fundamental differences between the two subtypes, probably having to do with the rapidity of progression of brain atrophy in patients with PPMS.”
The analysis was performed using data from the phase 2 SPRINT-MS trial, which was published in the New England Journal of Medicine in 2018 and for which Dr. Fox served as national principal investigator. The multicenter randomized trial found that ibudilast slowed progression of brain atrophy by nearly half compared with placebo over two years among patients with progressive forms of MS.
“The SPRINT-MS trial was unusual in that it enrolled people with both SPMS and PPMS,” explains Dr. Fox. “Most trials enroll just one type or the other, but we thought that if the subtypes were basically similar, it didn’t matter if we included both and it would help us enroll the trial in half the time it would otherwise.”
By chance, the study sample ended up being about half patients with PPMS (n = 134) and half patients with SPMS (n = 121), which gave the researchers an opportunity to answer a question they had been pondering for some time: Is treatment response similar between the two types of progressive disease?
Advertisement
The findings suggest not. The post hoc analysis looking at the drug’s effects by type of progressive disease found that the overall benefit of ibudilast was driven predominantly by its effect among patients with PPMS (P = 0.005) versus those with SPMS (P = 0. 97).
“Notably, when we looked solely at the two placebo groups — the placebo recipients who had PPMS versus those who had SPMS — we saw that atrophy progression was much faster in primary MS than in secondary MS,” Dr. Fox observes. That led the researchers to speculate that the between-group difference in treatment effect was at least partly precipitated by faster atrophy progression in the PPMS placebo group compared with the SPMS placebo group (P = 0.016).
When the researchers adjusted for various potential confounders, such as age, the difference in treatment effect trended toward statistical significance (P = 0.07) and was still driven by PPMS (P = 0.007).
Dr. Fox is quick to point out an important caveat: “Just because there was an impact on atrophy progression in the PPMS subjects and not in the SPMS subjects doesn’t mean ibudilast will have a differential effect on clinical disability in the two groups. These results suggest that PPMS might be a better treatment target than SPMS, but a phase 3 trial will be needed to evaluate whether ibudilast slows progression in either PPMS or SPMS.”
Such a trial is not yet underway. “The National Institutes of Health and the National MS Society funded SPRINT-MS, but they don’t have the resources to proceed to a phase 3 trial,” Dr. Fox notes. “Such a study would cost over $100 million and almost certainly need to be funded by the pharmaceutical industry. We hope that will happen soon.”
Advertisement
Image at top shows corticospinal tracts from a diffusion tensor imaging study acquired in SPRINT-MS.
Advertisement
Advertisement

New study advances understanding of patient-defined goals

Testing options and therapies are expanding for this poorly understood sleep disorder
Real-world claims data and tissue culture studies set the stage for randomized clinical testing
Digital subtraction angiography remains central to assessment of ‘benign’ PMSAH
Cleveland Clinic neuromuscular specialist shares insights on AI in his field and beyond

Findings challenge dogma that microglia are exclusively destructive regardless of location in brain

Neurology is especially well positioned for opportunities to enhance clinical care and medical training
New review distills insights from studies over the past decade