New grant will fund work to map human bladder and ureter at a cellular and molecular level
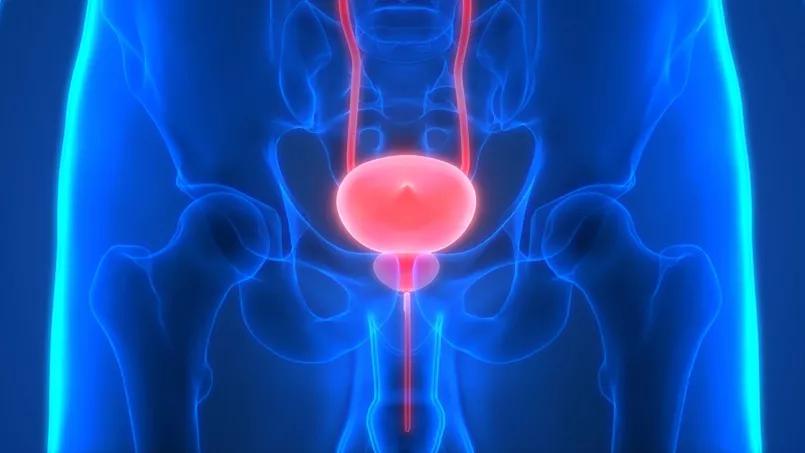
A multidisciplinary team of researchers from Lerner Research Institute has been awarded a five-year, $2.4 million grant from the National Institute of Diabetes and Digestive and Kidney Diseases (part of the National Institutes of Health) to conduct the first-ever comprehensive analysis of the human bladder and ureter throughout the lifespan.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
Non-cancerous urologic diseases (e.g., urinary tract infections, urinary incontinence and painful bladder syndrome) affect individuals of all ages, can lead to significant disability and decreased quality of life and cost more than $5 billion in healthcare expenditures each year in the United States.
“Development of effective novel therapies for these conditions has been hindered by our incomplete understanding of the lower urinary tract tissues at a cellular and molecular level,” says lead principal investigator Byron Lee, MD, PhD, joint staff in the Department of Cardiovascular & Metabolic Sciences and Glickman Urological and Kidney Institute.
“With this grant, we aim to generate a high-resolution transcriptome and gene regulatory dataset for healthy human ureter and bladder tissues in males and females at various ages. This will help us to construct an anatomical map of the organs that allows comprehensive understanding of their cellular and molecular characteristics during various life stages and points us closer to novel therapeutic targets.”
Using tissue samples from donors in three age groups that reflect distinct physiological states of lower urinary tract function, the researchers will collect data from five anatomic locations with unique tissue structures and functions. They then will use these data to analyze the lineage relationships between the different cell types, validate novel markers for each cell type and study underlying gene regulatory networks.
“These molecular data will become a valuable resource to the research community and, ultimately, will support efforts in organ repair and regeneration,” said Dr. Lee.
Advertisement
Angela Ting, PhD, associate staff in the Genomic Medicine Institute, and Oliver Wessely, PhD, associate staff in the Department of Cardiovascular & Metabolic Sciences, are also principal investigators on the grant.
Advertisement
Advertisement

Clinicians should individualize dosing practices based on patient risk factors and preferences
Pioneering and refining the approach in pyeloplasty, nephrectomy and more
Fully-automated process uses preop CT, baseline GFR to estimate post-nephrectomy renal function
Could mean earlier treatment, but also could have negative effects

Unlike earlier pills, new drugs do not cause liver toxicity

Male factors play a role in about half of all infertility cases, yet men often are not evaluated
Surgeons choreograph nearly simultaneous procedures, sharing one robot between two patients

Identifying barriers in the renal genetic assessment of Black patients