Studies seek new targets for treatment of retinopathies

Cleveland Clinic researchers are examining basic molecular mechanisms of angiogenesis as a key to eventual prevention and treatment of proliferative retinopathies linked to pathological neovascularization.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy

Vision in humans and animals relies on a clear path of light through the retina to the back of the eye. Abnormal growth and leakage of retina and choroid blood vessels cause a disruption of the light path that result in distorted vision. In the laboratory of ophthalmic researcher Bela Anand-Apte, MD, PhD, at Cleveland Clinic’s Cole Eye Institute, scientists are at work to understand the basic molecular mechanisms of angiogenesis and examine the physiological and pathological pathways that regulate retinal vision leakage.
Dr. Anand-Apte’s research focuses on the biology of how blood vessels in the eye (retina and choroid) develop, how they’re maintained and what causes them to grow abnormally and leak in diseases that result in loss of vision. “We need to evaluate the mechanisms that keep these blood vessels in check under normal conditions and identify factors that cause these normally quiescent vessels to go awry and start proliferating and leaking in disease states,” says Dr. Anand-Apte. Studies aimed at these targets will allow her lab to identify potential therapeutic approaches to prevent or reverse the effects of ocular neovascularization.
According to Dr. Anand-Apte, angiogenesis and leakage of retinal vessels are related to diseases that include diabetic retinopathy, retinal vein occlusions and retinopathy of prematurity in infants who are born before 30 weeks or weigh fewer than three pounds at birth.
Subretinal neovascularization involves abnormal growth of choroid vessels that break through Bruch’s membrane and leak into the subretinal space. Diseases that can result from this process include age-related macular degeneration (AMD), ocular histoplasmosis and pathologic myopia.
Advertisement
“These diseases are major causes of blindness,” says Dr. Anand-Apte, pointing out that pathological neovascularization plays a critical role in the development of proliferative retinopathies. “Our goal is to understand the mechanisms that underlie pathological neovascularization so that we can identify new targets for treatment,” she explains.
A major cause of pathological neovascularization in both the retina and choroid is an increase in vascular endothelial growth factor. This is the basis for current therapies such as Avastin®, Lucentis® and Eylea®. However, only about 30 percent to 40 percent of patients with AMD respond to these drugs. This indicates that there may be other unexplored mechanisms that could be potential drug targets.
Studies have shown that inactivation of a disintegrin and metalloproteinase 17 (ADAM17), a membrane- anchored metalloproteinase that regulates epidermal growth factor receptor (EGFR) signaling, reduces pathological retinal neovascularization. Tissue inhibitor of matrix metalloproteinases-3 (TIMP3) is an inhibitor of ADAM17.
According to Dr. Anand-Apte, a recent collaboration with Carl Blobel, MD, at the Hospital for Special Surgery, New York, determined that TIMP3 might mediate its antiangiogenic effects via ADAM17 and the EGFR.
These efforts have yet to pay off in clinical applications of research findings. Rather, they serve as the foundation for future treatments. “We’re at the stage of basic science,” says Dr. Anand-Apte, “but in time, our findings will lead to new ways to prevent, reverse or treat the diseases of retinal and choroidal neovascularization.”
Advertisement
Advertisement
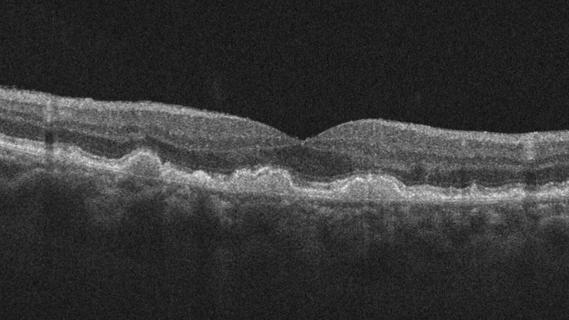
Early data shows risk is 73% higher in patients with lupus, 40% higher in patients with rheumatoid arthritis

Identifies weak spots in the cornea before shape change occurs
Study highlights the value of quantitative ultra-widefield angiography
Switching medications may decrease treatment burden and macular fluid

Interventions abound for active and stable phases of TED

Corneal imaging and interpretation play a major role
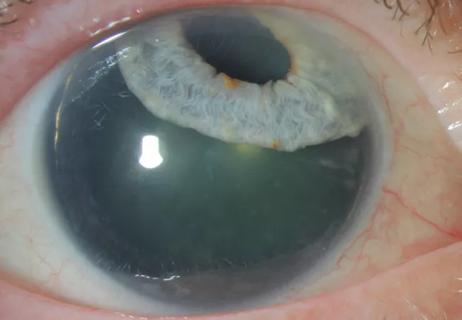
Cole Eye Institute imaging specialists are equal parts technician, artist and diagnostician
Effect of low-dose atropine and dual-focus contact lenses is unknown in patients with comorbid eye conditions