Cleveland Clinic among 24 Research Centers of Excellence designated by LBDA
Cleveland Clinic is among 24 U.S. academic medical centers comprising the new Research Centers of Excellence (RCOE) partnership launched April 3 by the Lewy Body Dementia Association (LBDA).
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
The RCOE program is designed to provide a coordinated research resource to support expanded efforts to conduct clinical trials related to Lewy body dementia (LBD) and provide expert clinical care to patients and their families and caregivers, according to the LBDA.
“We are very excited to be a Research Center of Excellence site, as designated by the Lewy Body Dementia Association,” says James Leverenz, MD, Director of Cleveland Clinic Lou Ruvo Center for Brain Health, Cleveland. “This program creates a consortium of sites ready for the rapid implementation of new treatment trials, thus accelerating the development of treatments for this devastating disease.”
LBD is one of the most common forms of neurodegenerative disease in the elderly, but it is believed to be underdiagnosed. This stems in part from its notoriously difficult diagnosis, as it shares symptoms with both Alzheimer’s disease and Parkinson’s disease.
Part of the rationale for the RCOE program is to coordinate the study of sufficient numbers of correctly diagnosed patients with LBD to shed more light on the disease and how best to manage it. The program will do so by establishing a clinical trials-ready network of leading institutions committed to providing top-level clinical care throughout the course of LBD management, according to the LBDA. Additional program goals include:
Advertisement
“Clinical trials with a patient population like LBD’s require experienced diagnosticians to insure accurate patient participation,” said Mike Koehler, CEO of the LBDA, in a statement. “This network can share a standardized approach to patient recruitment and data collection for clinical trials.”
According to the LBDA, the RCOEs were chosen for their clinical expertise in LBD, their experience running clinical trials for related conditions, their facility’s capacity and willingness to participate, and their geographic location. The 24 centers are distributed across 17 states and the District of Columbia and cover 23 metropolitan areas.
Each center is led by a recognized leading LBD investigator, the LBDA notes. “The RCOE program recognizes sites with the specific expertise needed for the diagnosis and management of patients with LBD,” says Dr. Leverenz, who serves as the principal investigator for the Cleveland Clinic RCOE site and also directs the multicenter Dementia with Lewy Bodies Consortium funded by a five-year NIH grant (as detailed in this earlier Consult QD post).
“The RCOE will collaborate on industry-sponsored clinical trials and apply for federal funding for LBD research initiatives,” noted the LBDA in a statement announcing the RCOE program launch. “As our program evolves, LBDA hopes to fund our own clinical research initiatives as we move forward.”
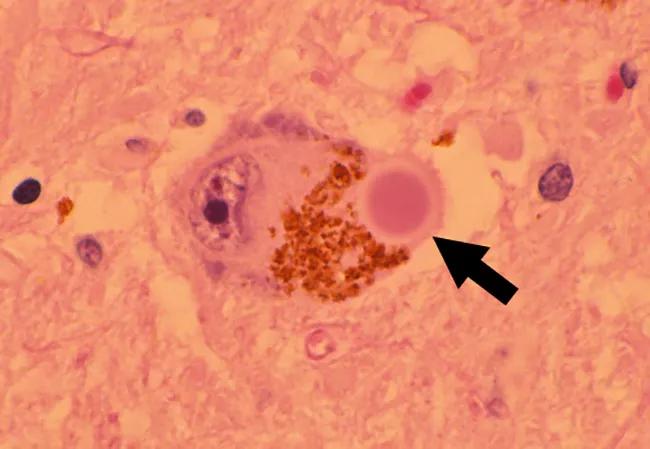
Image at top: Lewy body inclusion (arrow) in a pigmented neuron of the substantia nigra.
Advertisement
Advertisement

New study advances understanding of patient-defined goals

Testing options and therapies are expanding for this poorly understood sleep disorder
Real-world claims data and tissue culture studies set the stage for randomized clinical testing
Digital subtraction angiography remains central to assessment of ‘benign’ PMSAH
Cleveland Clinic neuromuscular specialist shares insights on AI in his field and beyond

Findings challenge dogma that microglia are exclusively destructive regardless of location in brain

Neurology is especially well positioned for opportunities to enhance clinical care and medical training
New review distills insights from studies over the past decade