Continued benefit on MRI activity without first-dose cardiac effects
Efficacy and safety of the investigational therapy ozanimod were maintained out to two years in the blinded extension of the placebo-controlled phase 2/3 RADIANCE trial in patients with relapsing multiple sclerosis (MS). So reported lead investigator Jeffrey A. Cohen, MD, in a scientific platform presentation at the American Academy of Neurology’s 2017 annual meeting on April 25.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
“These extension results confirm the benefit on MRI lesion activity we saw in the six-month core RADIANCE study with continued good tolerability,” says Dr. Cohen, Director of Cleveland Clinic’s Mellen Center for Multiple Sclerosis Treatment and Research. “To date, ozanimod is showing clinical efficacy that appears similar to that of fingolimod with an improved safety profile.”
The comparison with fingolimod, the first FDA-approved oral therapy for MS, stems from the fact that both agents are members of the sphingosine 1-phosphate (S1P) receptor modulator drug class. Ozanimod, also given orally, was developed as a somewhat more selective S1P modulator in hopes of mitigating some safety concerns with fingolimod, including the risk of first-dose cardiac effects.
Those hopes were initially supported in the phase 2 portion of the RADIANCE study (published in Lancet Neurology in April 2016), which showed ozanimod to significantly reduce MRI lesion activity relative to placebo while demonstrating a favorable safety profile over six months of therapy.
RADIANCE was designed prospectively as a combined phase 2/3 trial to allow interim analysis during the phase 2 portion and enable phase 3 to proceed more quickly. The trial’s newly reported blinded extension, which continued through two years of therapy, had three objectives:
Advertisement
The trial enrolled patients with active relapsing MS from 13 countries (the U.S. plus 12 European nations) and initially randomized them to one of three arms:
Of the 252 patients completing the core six-month study, 249 entered the blinded extension, and 224 of these patients (90 percent) completed the full extension period (two years of follow-up). Patients initially randomized to ozanimod continued with their assigned dosage, but original placebo recipients were re-rerandomized after the core six-month study to one of the two ozanimod doses.
The primary end point was cumulative number of total gadolinium-enhancing MRI lesions at 48 weeks and 96 weeks of therapy.
Results of the extension revealed that ozanimod continued to reduce mean gadolinium-enhancing lesions and mean new/enlarging T2-weighted lesions in a dose-dependent manner at both 48 weeks and 96 weeks.
Both ozanimod treatment groups maintained reductions in unadjusted annualized relapse rates, as follows:
No evidence of disease activity was observed at the following rates in the two treatment arms:
Three-quarters of patients had at least one adverse event, with comparable rates observed across treatment groups. None of the 20 serious adverse events was judged to be related to ozanimod. There were no reports of first-dose cardiac effects (including bradycardia or atrioventricular block) or macular edema. Elevated alanine aminotransferase levels were observed in 4.4 percent of patients, with similar distribution among dosage arms and no cases approaching Hy’s law criteria.
Advertisement
“The extension didn’t yield big surprises,” Dr. Cohen notes. “Efficacy was maintained over two years, no safety concerns were identified and patients who were transferred from placebo to ozanimod recapitulated the benefit observed in the core study, with reductions in their relapse rate and MRI lesion activity.”
He adds that two new insights emerged. First, ozanimod’s benefit appeared to become more pronounced over time. “This is not unexpected, since these agents don’t work instantly and downstream clinical effects will manifest more fully with time,” he says. Second, the dose effect hinted at in the core study became more prominent. “The 1.0-mg dose seemed to be somewhat more effective without a difference in safety.”
Dr. Cohen says the ongoing pivotal phase 3 RADIANCE and SUNBEAM studies should determine whether these promising findings translate to “a convincing benefit in terms of relapses and, hopefully, disability accrual.” These trials will also assess ozanimod’s safety profile across a broader patient population.
Longer-term questions include how ozanimod compares with other selective S1P receptor modulators under investigation. “Ozanimod has some pharmacologic properties that allow us to titrate the dose when starting therapy, which helps lessen the risk of first-dose cardiac effects seen with fingolimod,” Dr. Cohen notes. “Other S1P receptor modulators have some of the same advantages, although other pharmacologic properties could be distinguishing.”
Advertisement
Advertisement

New study advances understanding of patient-defined goals

Testing options and therapies are expanding for this poorly understood sleep disorder
Real-world claims data and tissue culture studies set the stage for randomized clinical testing
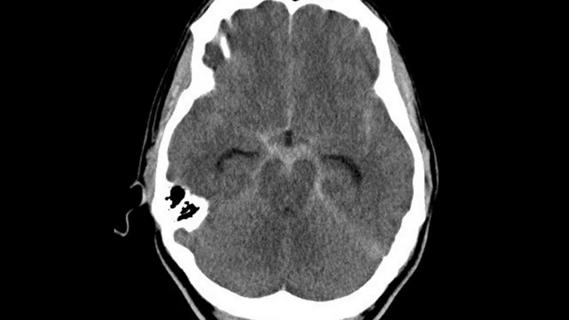
Digital subtraction angiography remains central to assessment of ‘benign’ PMSAH
Cleveland Clinic neuromuscular specialist shares insights on AI in his field and beyond

Findings challenge dogma that microglia are exclusively destructive regardless of location in brain

Neurology is especially well positioned for opportunities to enhance clinical care and medical training
New review distills insights from studies over the past decade