One of the largest-ever device trials will assess antibiotic delivery system
As more and more older patients with comorbidities receive cardiovascular implantable electronic devices (CIEDs), recognition of associated complications has risen, and infection has emerged as perhaps the most important. Device-related infections are associated with significant morbidity and mortality. They cannot be cleared while the device or its leads remain in the body, and device and lead removal is indicated.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
At this time, little can be done to minimize or reduce infection risk: Only preoperative or perioperative antibiotics have been shown to help. Cleveland Clinic electrophysiologists are hoping an antibiotic-permeated, bioabsorbable, implantable CIED envelope will be effective in reducing infection.
The FDA approved Medtronic’s TYRX™ antibacterial envelope in June 2013 based on retrospective and prospective nonrandomized studies. Since then, adoption of the envelope has been slow. Cleveland Clinic has worked with Medtronic to launch an international randomized controlled trial that will enroll up to 7,000 subjects at 225 sites, making it one of the largest medical device trials ever.
“Previous case-control and single-arm studies suggest this absorbable antibacterial envelope may be effective at preventing CIED infection,” says Cleveland Clinic electrophysiologist Bruce Wilkoff, MD, the study chair and president of its steering committee. “However, there may be unrecognized problems that would show up in a randomized controlled trial.”
The study — known as the Worldwide Randomized Antibiotic Envelope Infection Prevention Trial (WRAP-IT) — is a randomized, prospective, single-blind, multisite, postmarket interventional trial with important outcome measures. Its primary purpose is to evaluate the antibacterial envelope’s ability to reduce major CIED infections within 12 months after replacement, upgrade or revision of the CIED generator or de novo defibrillator implant.
“Nobody wants to do a device change or upgrade and have the patient return with infection,” says Khaldoun Tarakji, MD, MPH, who serves as global principal investigator of WRAP-IT. “It’s frustrating when you do everything by the book and this continues to happen.”
Advertisement
The trial will also serve as a postapproval study for the FDA and other regulatory bodies around the world requiring it.
The design of WRAP-IT allows physicians to use the envelope in standard device implantation procedures, in the hope of determining how real-world situations contribute to infection rates.
“Different institutions use different techniques and measures to minimize infection, and there are no clear guidelines that say one approach is better than another,” Dr. Tarakji explains. “We’re hoping WRAP-IT will enable us to examine the correlation between different practices and rates of infection.”
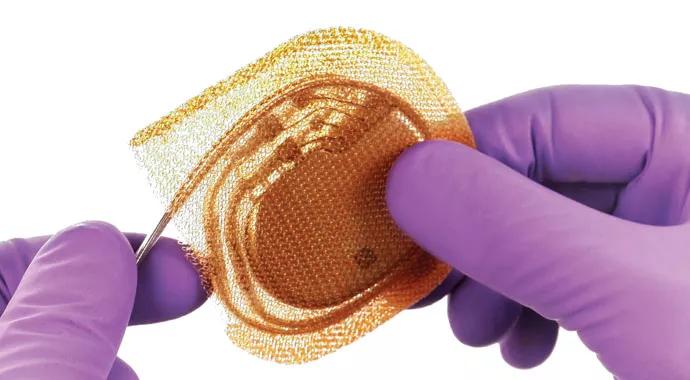
The antibiotic envelope is woven from fully bioabsorbable filament (figure) similar to resorbable sutures and coated with a bioabsorbable polymer containing two antibiotics, minocycline and rifampin. A nonrandomized observational study presented at the Heart Rhythm Society’s 2013 scientific sessions showed a reduction in infections of up to 90 percent with use of these same two antibiotics.

Figure. The antibacterial envelope is woven from filament that holds the CIED in place while eluting antibiotics for several days before eventually being absorbed by the body. Images (including image at top of post) courtesy of Medtronic.
The pulse generator and residual leads are placed into the envelope, which is then placed into the tissue pocket. The envelope elutes the antibiotics over a minimum of seven days, delivering less than 10 percent of the recommended daily oral dose of each agent. The envelope is fully absorbed by the body about nine weeks after implantation.
Advertisement
Enrollment is expected to begin in 2015 and continue through the first half of 2016. The final study report is expected in December 2017.
“In addition to wreaking havoc clinically, infections are expensive,” says Dr. Wilkoff. “This trial will reveal the true rate of infection around the world, as well as whether this device reduces that rate and is cost-effective. If the study shows a reduction in infections by 50 percent or more, the envelope will be incorporated into practice guidelines and be adopted on a wide scale.”
Advertisement
Advertisement

A sampling of outcome and volume data from our Heart & Vascular Institute
Concomitant AF ablation and LAA occlusion strongly endorsed during elective heart surgery
Large retrospective study supports its addition to BAV repair toolbox at expert centers
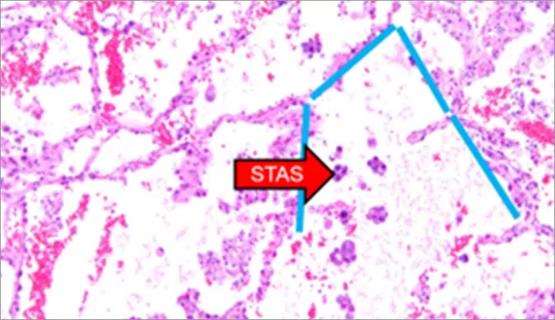
Young age, solid tumor, high uptake on PET and KRAS mutation signal risk, suggest need for lobectomy
Surprise findings argue for caution about testosterone use in men at risk for fracture
Residual AR related to severe preoperative AR increases risk of progression, need for reoperation

Findings support emphasis on markers of frailty related to, but not dependent on, age
Provides option for patients previously deemed anatomically unsuitable