Interim analysis from a second major trial shows success
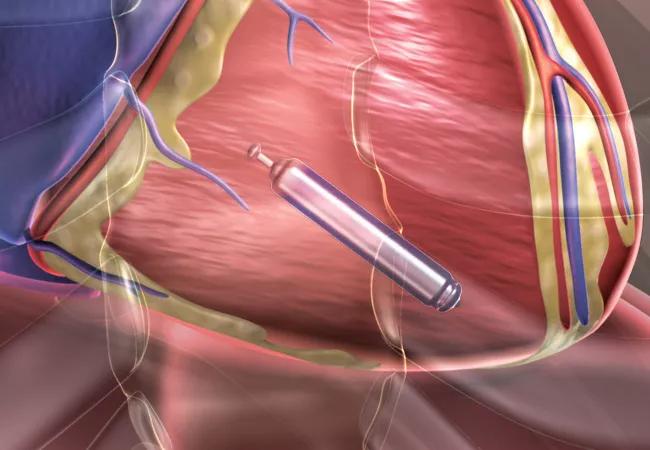
For the second time in a few months, a major multicenter clinical trial has demonstrated the feasibility of leadless pacemakers, a promising and rapidly advancing technology that some call the future of cardiac pacing.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
Interim data from an ongoing trial recently published in New England Journal of Medicine show efficacy and safety for Medtronic’s Micra™ Transcatheter Pacing System. Those results follow a similarly successful interim analysis of St. Jude Medical’s Nanostim™ leadless pacemaker, announced in August 2015 and covered previously on Consult QD.
“This is the future — no question about it,” says Cleveland Clinic electrophysiologist Khaldoun Tarakji, MD, MPH. “It’s a major step.”
Both devices are approved in Europe, and the companies developing each device are now working with the FDA to bring them to the U.S. market. Cleveland Clinic served as a study site for both trials.
“Leadless pacing is a promising avenue,” says Dr. Tarakji’s colleague Daniel J. Cantillon, MD. “Cleveland Clinic is committed to further advancement of this technology insofar as it benefits our patients.”
Traditional cardiac pacemakers consist of electrical generators connected to transvenous leads that deliver the pacing therapy to the heart. While the devices are effective, about 1 in 8 patients develops complications such as dislodgement of a lead or infection in the subcutaneous pocket that is surgically created to house the device.
Leadless pacing is now possible thanks to advances in battery technology that allow for a fully self-contained pacemaker small enough to be placed directly within the heart.
“Leadless pacemakers are exciting because you don’t have leads and you don’t have a pocket, so you eliminate the majority of complications associated with the traditional pacemaker,” Dr. Tarakji says.
Advertisement
The leadless devices are intended for use in patients who require single-chamber ventricular demand pacing, who represent about 10 percent of all pacemaker candidates.
The Micra study, a prospective noncontrolled investigation of 725 patients with indications for ventricular pacing, is the largest leadless pacemaker trial to date. Using a femoral vein approach to direct the device to the right ventricle, operators from 56 centers worldwide successfully implanted the device in 719 patients (99 percent).
“For a brand-new technology, it is remarkable how successful the implantation has been,” Dr. Tarakji notes.
The primary efficacy end point — achievement of low and stable pacing capture thresholds at six months after implantation — was met in 98.3 percent of patients, and the primary safety endpoint — freedom from major complications related to the system or procedure — was met in 96.0 percent.
Of the 28 major complications that occurred in 25 of the 725 patients, the most common were cardiac injuries (n = 11), groin puncture site complications (n = 5) and thromboembolism (n = 2). There were no device dislodgements.
The overall freedom-from-complication rate of 96 percent compared favorably with the prespecified 83 percent rate for traditional pacemakers from six previously published studies (P < .001), yet Dr. Tarakji notes that it’s a bit of an apples-to-oranges comparison. “The complications are completely different,” he says. “You can’t talk about lead dislodgement or pocket infection if you don’t have a lead or a pocket. At the same time, this new technology can lead to new complications, including venous access complications.”
Advertisement
He adds that the procedure also differs from placement of traditional pacemakers: “Technically the leadless implantation is easier, but there’s still a learning curve.”
These interim results with the Micra device are roughly comparable to those in the interim analysis of the Nanostim study, in which 90 percent of the 300 patients met efficacy end points and 93 percent met safety end points.
The devices differ in some features, as the Micra permanently attaches to the myocardium with tines (clips), whereas the Nanostim uses a retrievable helix. While the Micra is smaller in size, the Nanostim has a longer battery life.
But the two devices haven’t been studied head-to-head, and both will likely evolve, Dr. Cantillon points out. “With any new technology, what we typically see over time is if there’s a particular mechanism that’s better, the technologies tend to converge to whatever is safer and most effective,” he notes.
And while current leadless pacemaker technology can be used only in patients who require single-chamber right ventricular pacing, Dr. Cantillon expects future leadless pacing devices to expand to single-chamber pacing in the upper chambers of the heart — and ultimately to dual-chamber pacing.
“As with any new technology, there’s always cautious optimism and careful attention to the details involved, particularly with regard to long-term issues such as battery replacement,” he says. “That said, we’re very enthusiastic about this technology and look forward to seeing it grow.”
Advertisement
Advertisement

A sampling of outcome and volume data from our Heart & Vascular Institute
Concomitant AF ablation and LAA occlusion strongly endorsed during elective heart surgery
Large retrospective study supports its addition to BAV repair toolbox at expert centers
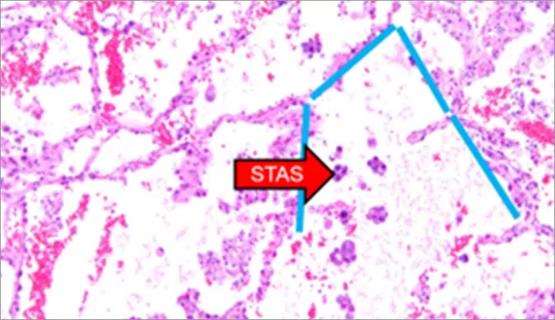
Young age, solid tumor, high uptake on PET and KRAS mutation signal risk, suggest need for lobectomy
Surprise findings argue for caution about testosterone use in men at risk for fracture
Residual AR related to severe preoperative AR increases risk of progression, need for reoperation

Findings support emphasis on markers of frailty related to, but not dependent on, age
Provides option for patients previously deemed anatomically unsuitable