Designed for infants weighing as little as 5 kg
Cleveland Clinic researchers have been the first to demonstrate successful implantation of a pediatric continuous-flow total artificial heart (P-CFTAH) in an acute animal study. The results are from a series of implantations of the Cleveland Clinic-developed P-CFTAH in four healthy lambs reported at the American Heart Association (AHA) Scientific Sessions in November 2017.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
The P-CFTAH is a downscaled version of Cleveland Clinic’s CFTAH for adult patients (previously reported on in 2015), with size reductions of 30 percent in all dimensions to allow implantation in children and infants with a body surface area as small as 0.3 m2, or approximately 5 kg body weight.
The device (illustrated below) features a dual-pump design (two valveless continuous-flow pumps) unified in a single, continuously rotating brushless DC motor and rotor assembly supported by a hydrodynamic bearing. Its sensorless design can produce pulsatile flow with speed modulation and automatically balance left and right circulations without electronic intervention.
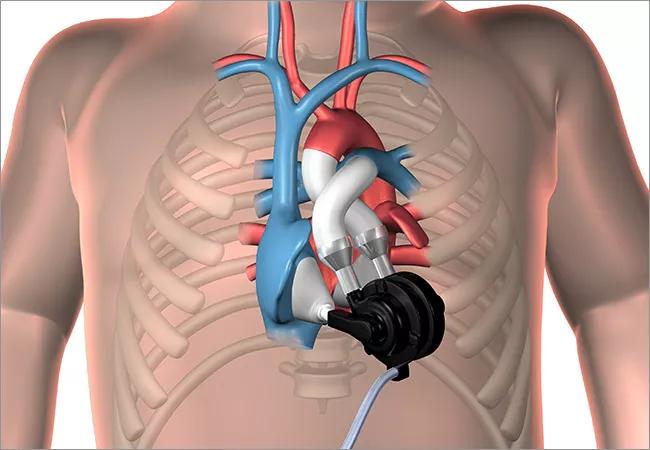
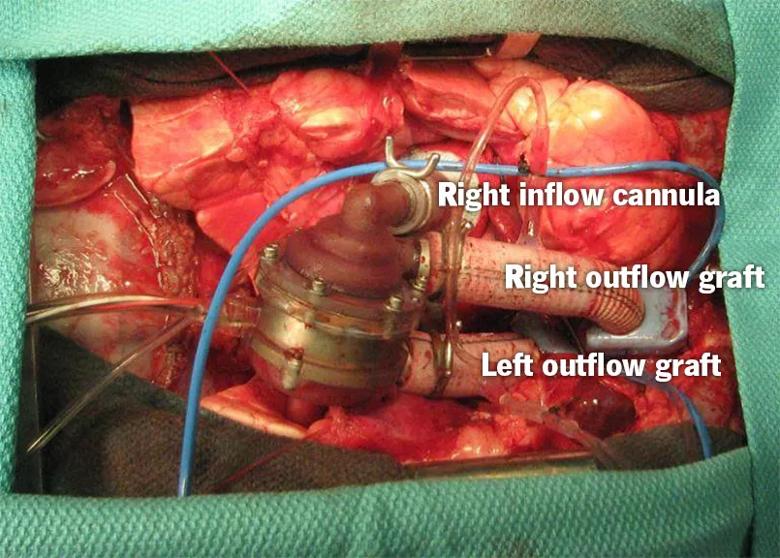
Implantation in the acute animal study (see photo below) was done through a full median sternotomy. Mean weight of the lambs was 28.7 ± 2.3 kg. All four experimental procedures demonstrated good anatomical fit and easy implantation, with a mean aortic cross-clamp time of 98 ± 18 minutes.
The P-CFTAH’s self-regulating performance — including pump speed, flow and current — was confirmed in all animals. Standard hemodynamic measures (arterial pressure and left and right atrial pressures) remained stable, and good left and right atrial balance was maintained in response to manipulations of systemic and pulmonary vascular resistance. No pump failures occurred. The duration of support ranged from 56 to 149 minutes (mean, 90 minutes).
“The early P-CFTAH prototype implantation was successfully performed in a small-size acute animal model, and the device performed within intended design specifications,” says Jamshid Karimov, MD, PhD, of the Department of Biomedical Engineering in Cleveland Clinic Lerner Research Institute, who presented the findings at the AHA meeting.
Advertisement
Device size is critically important for appropriate fit of the device in small children. “Our early testing has validated the feasibility of the dimensional scaling of this device in regard to size and performance,” adds principal investigator Kiyotaka Fukamachi, MD, PhD.
Scaling is a critical area of investigation, as only one other experimental TAH currently exists for patients with a small body surface area (BSA), and that device is intended only for patients with a BSA of 1.2 m2 (approximately 35 kg body weight) or larger, compared with 0.3 m2 (about 5 kg) or larger for the P-CFTAH.
“The design of the P-CFTAH allows for a significant size reduction while forgoing the use of valves or flow sensors for control,” notes Dr. Fukamachi. “This smaller, simpler pump design promises to increase system reliability.”
Further optimization of the device is needed to ensure biocompatibility and long-term balancing and regulation of flow and pressure, Dr. Karimov notes.
“If durability of the P-CFTAH is proven in eventual clinical testing, this device could be used as both a bridge to transplant and as destination therapy,” says Hani Najm, MD, Chair of Pediatric and Congenital Heart Surgery at Cleveland Clinic. “That would help address substantial unmet needs among children listed for heart transplantation, who face the highest waiting-list mortality in solid-organ transplant medicine.”
“Because the P-CFTAH is being developed and tested in conjunction with Cleveland Clinic’s CFTAH for adults, this research collectively aims to cover the majority of patients via two pump sizes,” adds pediatric and congenital heart surgeon Robert Stewart, MD. “The overlap in performance would ideally allow a rapidly growing child to ‘graduate’ from the P-CFTAH to the CFTAH.”
Advertisement
Advertisement

A sampling of outcome and volume data from our Heart & Vascular Institute
Concomitant AF ablation and LAA occlusion strongly endorsed during elective heart surgery
Large retrospective study supports its addition to BAV repair toolbox at expert centers
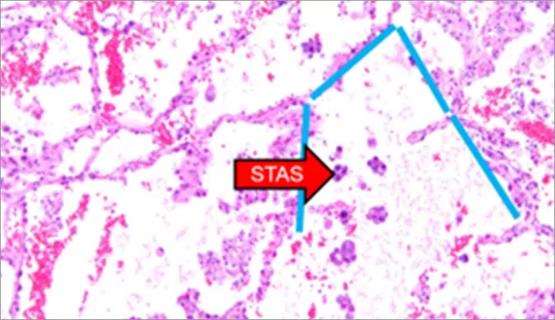
Young age, solid tumor, high uptake on PET and KRAS mutation signal risk, suggest need for lobectomy
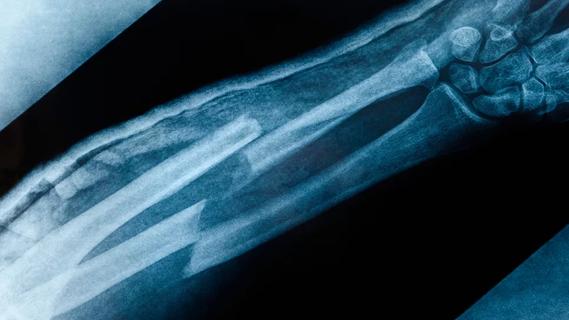
Surprise findings argue for caution about testosterone use in men at risk for fracture
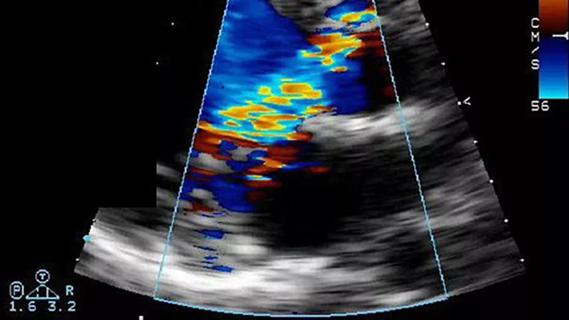
Residual AR related to severe preoperative AR increases risk of progression, need for reoperation

Findings support emphasis on markers of frailty related to, but not dependent on, age
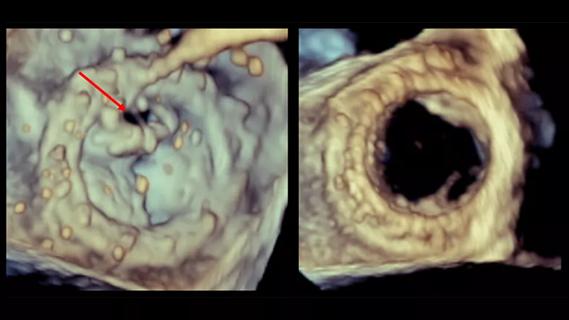
Provides option for patients previously deemed anatomically unsuitable