The following studies are either currently enrolling new patients or are pending approval by the Institutional Review Board and should be enrolling shortly:

A Prospective, Two-Cohort, Single-Masked Study to Evaluate the Effect of ESBA1008 Applied by Microvolume Injection or Infusion in Subjects with Exudative Age-Related Macular Degeneration
Objective: Demonstrate a treatment effect of ESBA1008 applied as microvolume injection or infusion on retinal function and morphology in subjects with exudative AMD.
Contact: Rishi Singh, MD, 216.445.9497, or Kathi Dastoli, RN, 216.445.5248
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
Ozurdex for Diabetic Macular Edema Treated with Pars Plana VitrEctomy and Membrane RemovAl (OPERA Study)
Objective: Examine the use of Ozurdex® (dexamethasone intravitreal implant) in patients who are undergoing pars plana vitrectomy for macular edema due to diabetic macular edema.
Contact: Sunil Srivastava, MD, 216.636.2286 or Kim Baynes, 216.444.2566
Safety and Efficacy of Intravitreal Ranibizumab for Diabetic Macular Edema Previously Treated with Intravitreal Bevacizumab: A Randomized Dual-Arm Comparative Dosing Trial (Phase: 1/2): REACT Study
Objective: Assess the ocular and systemic adverse events of ranibizumab for DME following previous treatment with intravitreal bevacizumab.
Contact: Justis Ehlers, MD, 216.636.0183, or Kathi Dastoli, RN, 216.445.5248
A Phase III, Randomized, Double-Masked, Controlled Trial to Establish the Safety and Efficacy of Intravitreous Administration of Fovista® (Anti PDGF-B Pegylated Aptamer) Administered in Combination with Lucentis Compared to Lucentis Monotherapy in Subjects with Subfoveal Neovascular Age-Related Macular Degeneration
Objective: Evaluate the safety and efficacy of Fovista intravitreous administration.
Contact: Rishi Singh, MD, 216.445.9497, or Kathi Dastoli, RN, 216.445.5248
Randomized, Double-Masked, Vehicle-Controlled, Clinical Evaluation to Assess the Safety and Efficacy of Nepafenac Ophthalmic Suspension, 0.3%, for Improvement in Clinical Outcomes Among Diabetic Subjects Following Cataract Surgery
Objective: Demonstrate superiority of nepafenac ophthalmic suspension, 0.3%, dosed once daily relative to nepafenac vehicle based upon clinical outcomes among diabetic subjects following cataract surgery.
Contact: Richard Gans, MD, 216.444.0848, or Kathi Dastoli, RN, 216.445.5248
Advertisement
Prevention of Macular Edema in Patients with Diabetic Retinopathy Undergoing Cataract Surgery
Objective: Determine the safety and efficacy of intravitreal aflibercept injection in patients with diabetic retinopathy in the prevention of macular edema following cataract surgery.
Contact: Rishi Singh, MD, 216.445.9497, or Kathi Dastoli, RN, 216.445.5248
Prospective Intraoperative and Perioperative Ophthalmic Imaging with Optical Coherence Tomography (PIONEER Study)
Objective: Assess the feasibility and utility of intraoperative OCT and perioperative OCT in optimizing the management of surgical ophthalmic diseases.
Contact: Justis Ehlers, MD, 216.636.0183, or Jamie Reese, RN, 216.636.0183
A Randomized, Double-Masked, Placebo-Controlled Study of the Safety and Efficacy of Gevokizumab in the Treatment of Active Non-Infectious Intermediate, Posterior, or Pan-Uveitis (Eyeguard-A Study)
Objective: Demonstrate the superiority of gevokizumab compared to placebo in the treatment of subjects with active non-infectious intermediate, posterior or pan-uveitis. The safety of gevokizumab will also be assessed.
Contact: Careen Lowder, MD, 216.444.3642, or Laura Holody, 216.445.3762
A Randomized, Double-Masked, Placebo-Controlled Study of the Safety and Efficacy of Gevokizumab in the Treatment of Subjects with Non-infectious Intermediate, Posterior, or Pan-Uveitis Currently Controlled with Systemic Treatment (Eyeguard-C Study)
Objective: Demonstrate the superiority of gevokizumab compared to placebo in reducing the risk of recurrent uveitic disease in subjects with non-infectious intermediate, posterior or pan-uveitis currently controlled with systemic treatment. The safety of gevokizumab will also be assessed.
Contact: Careen Lowder, MD, 216.444.3642, or Laura Holody, 216.445.3762
Advertisement
Open-Label, Safety and Tolerability Study of Suprachoroidal Triamcinolone Acetonide via Microneedle in Subjects with Non-Infectious Uveitis
Objective: Evaluate the safety, tolerability and efficacy of an injection of triamcinolone acetonide (TA) into the SCS of human subjects using a microneedle.
Contact: Sunil Srivastava, MD, 216.636.2286, or Kim Baynes, 216.444.2566
A Phase III, Multinational, Multicenter, Randomized, Masked, Controlled, Safety and Efficacy Study of a Fluocinolone Acetonide Intravitreal (FAI) Insert in Subjects with Chronic Noninfectious Uveitis Affecting the Posterior Segment of the Eye (PSV-FAI-001)
Objective: Evaluate the safety and efficacy of an FAI insert in the management of subjects with chronic noninfectious uveitis affecting the posterior segment of the eye.
Contact: Careen Lowder, MD, 216.444.3642, or Laura Holody, 216.445.3762
Bilateral Lateral Rectus Recession versus Unilateral Recess-Resect for Intermittent Exotropia (IXT1)
Objective: Evaluate the effectiveness of bilateral lateral rectus muscle recession versus unilateral lateral rectus recession with medial rectus resection procedures for the treatment of strabismus.
Contact: Elias Traboulsi, MD, 216.444.4363, or Sue Crowe, RN, 216.445.3840
The Natural History of the Progression of Atrophy Secondary to Stargardt’s Disease: Prospective Observation
Objective: Assess the rate of progression of Stargardt’s disease by measuring the growth of macular atrophic lesions using fundus autofluorescence.
Contact: Elias Traboulsi, MD, 216.444.4363, or Meghan Marino, 216.445.7671
Advertisement
The Natural History of the Progression of Atrophy Secondary to Stargardt’s Disease: Retrospective Longitudinal Observational Study
Objective: Assess the rate of progression of Stargardt’s disease by measuring the growth of macular atrophic lesions using fundus imaging/visual field loss.
Contact: Elias Traboulsi, MD, 216.444.4363, or Meghan Marino, 216.445.7671
Molecular Genetics of Eye Diseases
Objective: Study the molecular genetics of ophthalmic disorders through the compilation of a collection of DNA, plasma and eye tissue samples from patients and from families with a broad range of eye diseases and malformations.
Contact: Elias Traboulsi, MD, 216.444.4363, or Meghan Marino, 216.445.7671
Genetics of Uveitis
Objective: Identify changes in genes that may lead to uveitis.
Contact: Sunil Srivastava, MD, 216.636.2286, or Kim Baynes, 216.444.2566
LASIK Flap Thickness and Visual Outcomes Using the WaveLight FS200 Femtosecond Laser
Objective: To evaluate the visual outcome, accuracy and predictability of LASIK flap thickness using the new WaveLight® FS200 femtosecond laser and compare these results to those obtained using the IntraLase™ FS60 femtosecond laser.
Contact: William J. Dupps Jr., MD, PhD, 216.444.8158, or Laura Holody, 216.445.2264
Long-Term Safety Follow-Up For Subjects Previously Implanted with the AcrySof® Cachet™ Phakic Lens in Clinical Studies C-02-23, C-02-40, C-03-21 and C-05-57
Objective: Estimate the annualized endothelial cell loss rate (for up to 10 years following date of implantation) of subjects previously implanted with the L-series AcrySof Cachet Phakic Lens from clinical studies.
Contact: William J. Dupps Jr., MD, PhD, 216.444.8158, or Laura Holody, 216.445.2264
Advertisement
The following study has completed patient enrollment in the last year at Cole Eye Institute and is in follow-up:
Investigator Initiated Observational Study of Intravitreal Aflibercept Injection for Exudative Age-Related Macular Degeneration Previously Treated with Ranibizumab or Bevacizumab
Visit clevelandclinic.org/eye for the latest in Cole Eye Institute research, innovations and clinical trials.
Advertisement
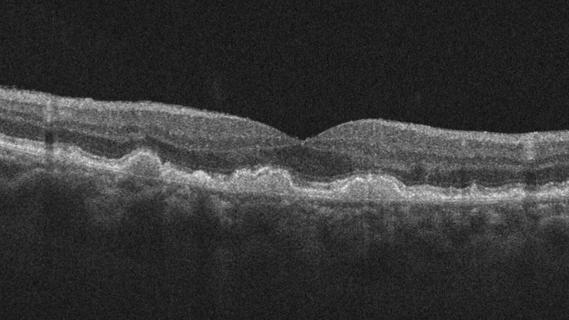
Early data shows risk is 73% higher in patients with lupus, 40% higher in patients with rheumatoid arthritis

Identifies weak spots in the cornea before shape change occurs
Study highlights the value of quantitative ultra-widefield angiography
Switching medications may decrease treatment burden and macular fluid

Interventions abound for active and stable phases of TED

Corneal imaging and interpretation play a major role
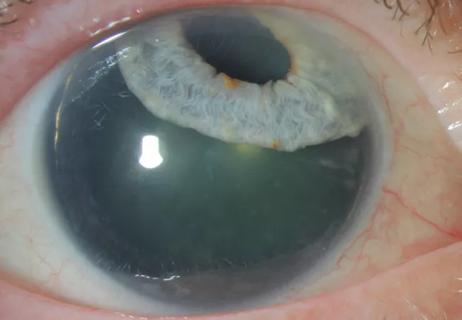
Cole Eye Institute imaging specialists are equal parts technician, artist and diagnostician
Effect of low-dose atropine and dual-focus contact lenses is unknown in patients with comorbid eye conditions