Quality indicators for four major endoscopic procedures to serve as a reference for doctors
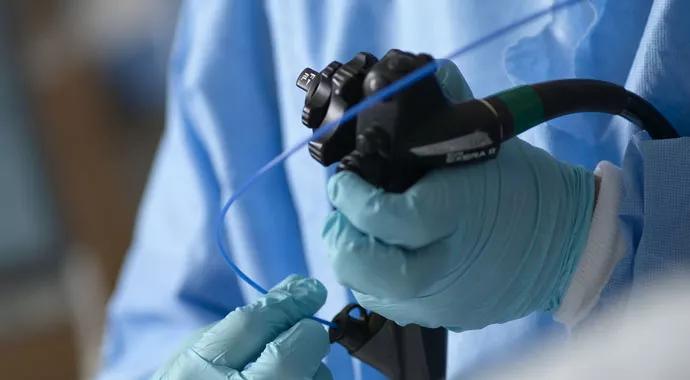
Physicians trained in endoscopy now have new guidelines to assess, measure and improve their outcomes.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
The new guidelines update quality indicators originally published in 2006 and aim to serve as a reference for doctors.
They were written by members of the ASGE/ACG Task Force on Quality in Endoscopy, a joint effort of the American Society for Gastrointestinal Endoscopy (ASGE) and the American College of Gastroenterology (ACG).
The task force decided take a new approach in writing the guidelines, says gastroenterologist Maged Rizk, MD, who served on the panel. Dr. Rizk is Quality Improvement Officer for the Digestive Disease Institute at Cleveland Clinic.
The group began by identifying quality metrics they consider important, then based on evidence, prioritized the metrics and set target rates.
As a result, the new guidelines not only seek to address minimum goals for each of the major endoscopic procedures, but also are aspirational in their intent to identify performance gaps. This will move continuous quality improvement for the entire field of endoscopy forward, Dr. Rizk says.
“The new guidelines pose questions that the endoscopy community could consider studying to develop and answer questions to improve the guidelines,” Dr. Rizk says. “Hopefully, we will have more outcome metrics and push the profession into the future.”
Major external forces stemming from policy makers, payers and patients have created demand for a way to accurately define and measure the quality of the services that endoscopists provide. The new guidelines will help to meet these demands, Dr. Rizk says.
Advertisement
“They can be assured that when a doctor performs an endoscopy, the physician is expected to adhere to a certain standard,” Dr. Rizk says. “Patients will know doctors are performing the right procedure for the right patient for the right reason.”
For physicians, the new guidelines can help them to maintain patient volume, but better match the patient to the procedure.
“Then a doctor can go to payers and say we perform at a higher quality level and we should be reimbursed as such,” Dr. Rizk says.
The guidelines are divided into five areas. One set of guidelines encompasses indicators common to all gastrointestinal endoscopic procedures. The other four cover indicators specific to the four major endoscopic procedures:
The articles appear in the January 2015 editions of GIE: Gastrointestinal Endoscopy, the official peer-reviewed scientific journal of ASGE, and The American Journal of Gastroenterology, the official peer-reviewed scientific journal of ACG.
An important goal of the new guidelines is to accurately define and measure the quality of the services that endoscopists provide, says Dr. Rizk, who led the group that authored the set of guidelines dealing with indicators common to all gastrointestinal endoscopic procedures.
For each endoscopic procedure, the authors considered quality indicators for three periods of time: preprocedure, intraprocedure and postprocedure.
Advertisement
The quality indicators are to help physicians ensure:
The guidelines also seek to ensure all of these steps are accomplished with minimum risk to the patient, Dr. Rizk says.
“We want to move from focusing on process to focusing on quality,” he says. “I don’t care how many times you take the picture. You want to know what the outcomes are.”
Shortly after the new guidelines were released, the CDC identified a cluster of carbapenem-resistant Enterobacteriaceae (CRE) cases linked to duodenoscopes used in ERCP.
Dr. Rizk says the new guidelines do not relate to the CRE outbreaks, although an updated white paper is expected in the next year that will discuss quality metrics for endoscopic units.
Dr. Rizk says that in the meantime, Cleveland Clinic’s medical staff is working to ensure that protocols for endoscopic units fully meet processing standards, including in-person observation and spot auditing.
“We additionally are working on measures beyond what the FDA would require, specifically using magnification technology to assess for bio-debris, and use of chemical testing for bio-debris,” he says.
Other quality initiatives in endoscopy under way at Cleveland Clinic include ensuring harmonization of protocols that ensure patient safety, Dr. Rizk says.
“In particular, we are making sure we are on the same page on blood thinners, specifically when and if they should be stopped, as well as anesthesia safety and minimizing our use of blood products,” he says.
Advertisement
For more information, contact Dr. Rizk at rizkm@ccf.org.
Advertisement
Advertisement

Strong patient communication can help clinicians choose the best treatment option

ctDNA should be incorporated into care to help stratify risk pre-operatively and for post-operative surveillance

The importance of raising awareness and taking steps to mitigate these occurrences
New research indicates feasibility and helps identify which patients could benefit

Treating a patient after a complicated hernia repair led to surgical complications and chronic pain
Standardized and collaborative care improves liver transplantations
Fewer incisions and more control for surgeons

Caregiver collaboration and patient education remain critical