Case reveals benefits of integrated pediatric and adult-care expertise
JM, a previously healthy 17-year-old male, presented to Cleveland Clinic with progressive headaches, nausea, vomiting, dizziness and weight loss. Echocardiogram showed a dilated noncompaction cardiomyopathy with a 19 percent shortening fraction.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
He was listed for heart transplantation, but before a match was found he developed an acute left hemispheric syndrome with sudden-onset unresponsiveness and a dense right hemiplegia. His NIH Stroke Scale (NIHSS) score was 23. Brain MRI and MR angiogram showed an acute deep infarct involving the left basal ganglia and corona radiata (Figure 1) secondary to a left M1 middle cerebral artery occlusion, with a large perfusion deficit involving much of the left hemisphere (Figure 2).
He underwent emergent intra-arterial acute stroke treatment with endovascular clot removal. Distal flow was re-established, and his post procedure NIHSS score was 1. He was restarted on heparin due to a left ventricular thrombus. Worsening heart failure required a left ventricular assist device, and he subsequently underwent a successful heart transplant. He has shown a remarkable recovery over the past three years and is doing well and attending college.
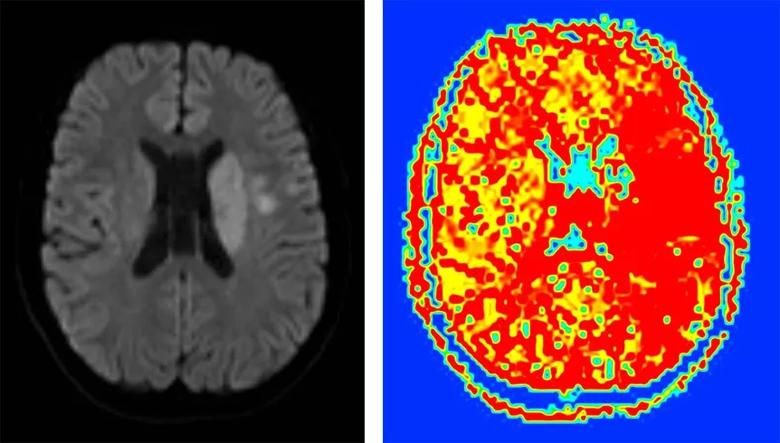
Figure 1 (left). Diffusion-weighted image showing the patient’s acute left basal ganglia and corona radiata infarct. Figure 2 (right). Perfusion image showing a deficit involving much of the left hemisphere.
The last few decades have seen a revolution in mechanical devices used in the treatment of adults with a variety of neurological conditions. Endovascular treatment with mechanical clot retrieval has been supported by class I, level A evidence in the treatment of adult arterial ischemic stroke and is now standard practice in adult stroke management. But experience remains limited in the use of mechanical devices for acute ischemic stroke revascularization in children, and no guidelines exist for acute revascularization in the pediatric population. Nevertheless, mechanical embolectomy continues to be used off-label, with several case reports over the past decade, and as illustrated by the case above. None of the major endovascular stroke trials have, to date, included pediatric populations.
Advertisement
While devices’ mechanism of action is generally expected to be similar in adults and pediatric patients — in contrast to what’s sometimes the case with medications — key differences exist that may limit devices’ application in children. Age-related differences in anatomy (vessel size), physiology (clotting system) and pathophysiology of pediatric stroke make direct application of devices specifically designed for adults a potential concern in children. Vessel-device size mismatch, for example, may increase rates of device-associated vasospasm, with potential risk of iatrogenic infarct. Other concerns include limitations on the size of catheters requiring femoral artery access, endothelial or vessel wall damage, and potential fragility of vessels.1
Despite a recognized need, relatively few devices have pediatric-specific indications, design or labeling. The FDA has recognized the challenges of developing medical devices labeled for pediatric use, including small and diffusely scattered potential pediatric populations, small trial sizes and challenges to enrollment and consent. Given this, a guidance document was released in 2016 aimed at leveraging adult clinical data for extrapolation to pediatric use.2 Similarly, the American Academy of Pediatrics has endorsed the off-label use of medical and surgical devices as “a necessary and appropriate part of care” given the clinical need for devices to diagnose and treat diseases in children and the reality that most devices do not have FDA clearance for use in pediatric populations.3
Advertisement
Clearly, the absence of testing for, and availability of, devices specifically for pediatric care poses ongoing challenges in the management of pediatric stroke and other pediatric neurological diseases, and draws attention to the chasm between adult and pediatric care.
In the face of these challenges, Cleveland Clinic has made strides in recent years through our ability to harness adult stroke expertise for application of these devices in pediatric stroke care. Cleveland Clinic’s status as a Joint Commission-certified Primary Stroke Center, along with ongoing close collaboration with our adult stroke colleagues, has allowed for an integrated and comprehensive stroke service between our pediatric and adult centers. Such integration facilitates 24/7 acute stroke management and care in children, with dedicated teams, emergency hotlines, in-house ICU staff-level care and deployment of the most modern technologies. This integrated approach, together with the development of a pediatric stroke protocol, has allowed us to maximize safety, quality and opportunities for pediatric care.
Ultimately, the establishment of certified pediatric stroke centers, equipped with pediatric-appropriate devices and expertise, should be the goal.4 In the meantime, we have the ability to offer children potentially lifesaving stroke management, given the significant advantages that emerge from collaboration between our adult and pediatric centers in a unified healthcare system.
Advertisement
Advertisement

New study advances understanding of patient-defined goals

Testing options and therapies are expanding for this poorly understood sleep disorder
Real-world claims data and tissue culture studies set the stage for randomized clinical testing
Digital subtraction angiography remains central to assessment of ‘benign’ PMSAH
Cleveland Clinic neuromuscular specialist shares insights on AI in his field and beyond

Findings challenge dogma that microglia are exclusively destructive regardless of location in brain

Neurology is especially well positioned for opportunities to enhance clinical care and medical training
New review distills insights from studies over the past decade