An expert’s take on FDA’s ‘appropriate rationale’ caveat

The FDA’s long-awaited approval earlier this year of the WATCHMAN™ left atrial appendage (LAA) closure device provides an alternative to oral anticoagulation therapy in high-risk patients with nonvalvular atrial fibrillation.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
The percutaneous LAA closure device is indicated to reduce the risk of thromboembolism in patients with nonvalvular afib who:
“Since FDA left the indication open to ‘appropriate rationale’ when considering these types of patients, it’s important to define what that is in order to weigh the risks and benefits and select the best candidates,” says Oussama Wazni, MD, Director of the Outpatient Electrophysiology Department and Co-Director of the Ventricular Arrhythmia Center in Cleveland Clinic’s Section of Cardiac Electrophysiology and Pacing.
In this video, Dr. Wazni discusses his clinical criteria for “appropriate rationale”: Patients with a history of bleeding when taking oral anticoagulants, or those whose future risk of bleeding is deemed to be high. He adds: “Implanting the WATCHMAN device in these patients would prevent stroke, while at the same time avoiding the risk of bleeding.”
Advertisement
Advertisement

A sampling of outcome and volume data from our Heart & Vascular Institute
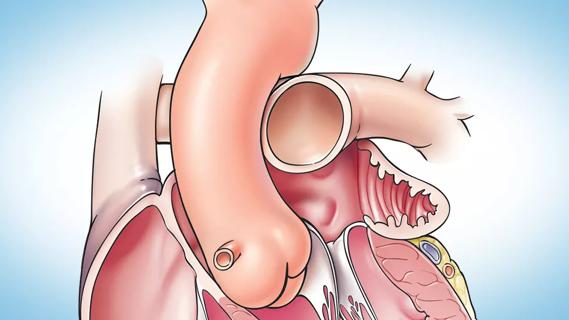
Concomitant AF ablation and LAA occlusion strongly endorsed during elective heart surgery
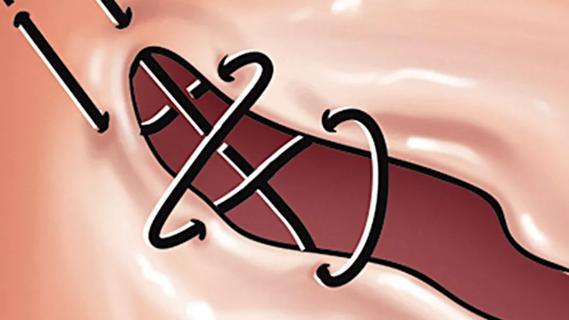
Large retrospective study supports its addition to BAV repair toolbox at expert centers
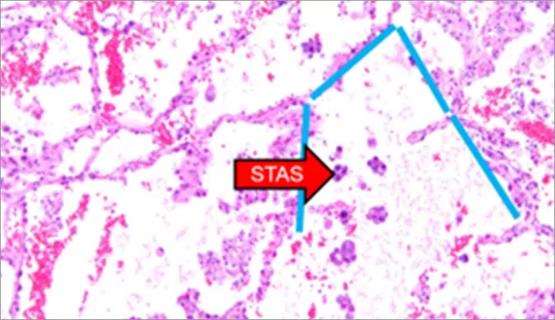
Young age, solid tumor, high uptake on PET and KRAS mutation signal risk, suggest need for lobectomy
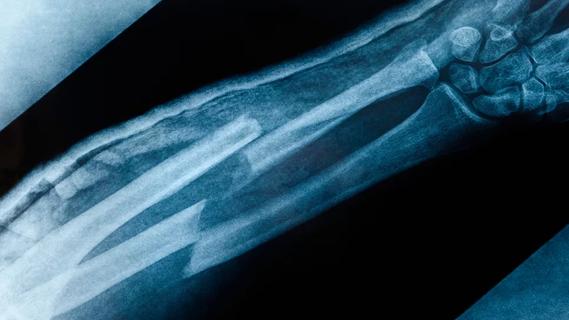
Surprise findings argue for caution about testosterone use in men at risk for fracture
Residual AR related to severe preoperative AR increases risk of progression, need for reoperation

Findings support emphasis on markers of frailty related to, but not dependent on, age
Provides option for patients previously deemed anatomically unsuitable