Efficacy and safety end points met in 90 percent of patients
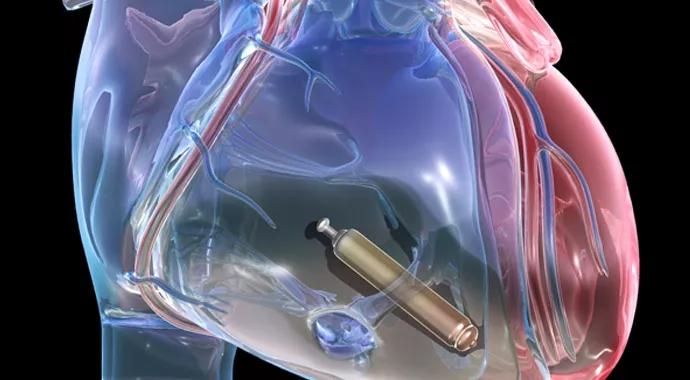
Interim data from the ongoing LEADLESS II study of an investigational leadless pacemaker were unveiled at the European Society of Cardiology meeting this week, and they appear to bode well for a new leadless era in cardiac pacemaker technology.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
“We’re seeing a shift in the field,” says Cleveland Clinic electrophysiologist Daniel J. Cantillon, MD, who was a co-investigator in the multicenter trial. “The weakest link in traditional pacemaker systems is the lead and the pocket. The leadless systems have neither, so in effect they break through that ceiling of limitations that have been in place for 60 years, and into a new era.”
The limitations he’s referring to are the short- and long-term complications from the transvenous leads that characterize traditional pacemakers, as well as infections that can develop within the pocket that’s created surgically to hold these devices. Over time, pacemaker-related adverse events occur in about 10 percent of patients who receive traditional pacemakers. Occasionally, manufacturers have needed to recall the devices due to lead-related problems.
A desire to eliminate those limitations prompted the development of miniaturized, fully self-contained leadless pacemakers, as detailed in this Consult QD story. Just one-tenth the size of traditional pacemakers, the new leadless pacemakers are implanted within the right ventricle nonsurgically using a catheter introduced from a vein in the leg.
“Now,” says Dr. Cantillon, “it’s a matter of developing and showing that these systems can be effective and safe and extend the benefit long-term.”
Demonstrating that for one investigational leadless pacemaker — St. Jude Medical’s Nanostim™ — is an objective of the ongoing prospective, nonrandomized LEADLESS II trial in which Cleveland Clinic is participating. In addition to being presented at the European Society of Cardiology meeting, its interim results are newly published in the New England Journal of Medicine.
Advertisement

The Nanostim™ leadless pacemaker that was studied in the trial. Image courtesy of St. Jude Medical.
According to the LEADLESS II researchers, the Nanostim was successfully implanted in 96 percent of 526 subjects, all of whom were selected because they were candidates for single-chamber ventricular pacing.
Of the 300 patients who had completed a minimum of six months of follow-up, 90 percent met prespecified endpoints for pacing (2.0 V or less at 0.4 msec) and sensing (R-wave 5.0 mV or greater), and 93 percent met the primary safety endpoint (freedom from serious device-related adverse events at six months).
Serious adverse events occurred in 6.7 percent of patients overall and included device dislodgement with successful percutaneous retrieval (1.7 percent), cardiac perforation (1.3 percent) and pacing capture threshold elevation requiring percutaneous retrieval and device replacement (1.3 percent).
Notably, when the first 10 device implantations for each operator were excluded from analysis, the serious adverse event rate dropped to 3.6 percent for the subsequent cases, a short-term complication rate approximately equivalent to that seen with traditional pacemakers.
“This is a completely brand-new technology,” Dr. Cantillon points out. “It’s significant that with experience in as few as 10 cases, operators who have never handled this technology before are able to approach adverse event rates that exist with traditional pacemakers.”
The main advantage to the leadless devices is anticipated to be seen over the long term, in part through avoidance of complications such as lead breakage, bleeding and infection in the surgical pocket, which occurs in about 2 to 3 percent of patients every time a traditional device’s generator needs to be replaced, typically at seven- to 10-year intervals.
Advertisement
Based on projections from the six-month follow-up data, investigators estimate that the Nanostim’s battery could last an average of 15 years, and replacement could be accomplished nonsurgically.
“Until you get away from the surgical pocket and the lead, you’re not going to make a leap forward,” Dr. Cantillon says. “This study is a big leap forward for the field.”
Once the study has been completed with the planned 667 subjects, St. Jude is expected to submit the data to the FDA as part of a new device application.
At the same time, work on refinements is ongoing. Although the Nanostim currently can be only used in patients requiring single-chamber pacing (who represent less than 10 percent of all patients with pacemakers), efforts are underway to develop dual-chamber and multisite-chamber pacing, Dr. Cantillon notes. “Our expectation is also that next generations will be safer and easier to handle, and represent an overall upgrade of what was evaluated in this trial.”
There’s also competition in the field. Medtronic also has a leadless pacemaker, the Micra™ Transcatheter Pacing System, which has already been licensed in Europe, as has St. Jude’s Nanostim.
“All the companies that make pacemakers are developing leadless pacemakers,” Dr. Cantillon observes. “We think this could be the future of pacing.”
Advertisement
Advertisement

A sampling of outcome and volume data from our Heart & Vascular Institute
Concomitant AF ablation and LAA occlusion strongly endorsed during elective heart surgery
Large retrospective study supports its addition to BAV repair toolbox at expert centers
Young age, solid tumor, high uptake on PET and KRAS mutation signal risk, suggest need for lobectomy
Surprise findings argue for caution about testosterone use in men at risk for fracture
Residual AR related to severe preoperative AR increases risk of progression, need for reoperation

Findings support emphasis on markers of frailty related to, but not dependent on, age
Provides option for patients previously deemed anatomically unsuitable