Exciting advances are improving outcomes and safety

The field of electrophysiology is abuzz with technological developments. Five recent advances have the potential to improve patient outcomes and make procedures safer.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
Two of these innovations—subcutaneous ICDs and contact force ablation catheters—recently received FDA approval. Cleveland Clinic electrophysiologists remain involved in clinical trials of other techniques and technologies that are expected to improve medical practice: leadless pacemakers, a vagal nerve stimulator and a left atrial appendage occlusion device.
“It’s exciting to be part of these clinical trials. As a physician, you always want to serve your patients in ways that are better and safer,” says Cleveland Clinic electrophysiologist Daniel Cantillon, MD.
With the lead and the surgical pocket being the weakest links in any pacing system, leadless pacemakers are expected to lower complication rates.
More than 116 patients in the U.S. and Canada—six at Cleveland Clinic—have received St. Jude Medical’s single-chamber Nanostim device to date through the LEADLESS II trial.
“Our experience at Cleveland Clinic has been quite favorable, but it’s still early,” says Dr. Cantillon, who serves on the steering committee for the North American trial and as principal site investigator. “Our endpoint is six months’ freedom from complications. Since our first Nanostim was implanted on Valentine’s Day, we are just approaching that six-month mark.”
Cleveland Clinic will also participate in clinical trials of a second leadless pacemaker, Medtronic’s single-chamber Micra device, with Bruce Wilkoff, MD, as site investigator. Patient enrollment should begin soon.
With new treatments for heart failure few and far between, all eyes are focused on CardioFit. This pacemaker-style device is the first to successfully activate the autonomic nervous system in order to improve heart failure.
Advertisement
The device is implanted under the skin, with one electrode leading to the vagus nerve and the other secured in the heart muscle. The device senses heart rate and delivers a stimulus to the vagus nerve to mitigate harmful changes that occur as part of the heart failure disease process. In a European pilot study, the system improved 6-minute walk distance, left ventricular ejection fraction and heart failure symptoms (NYHA class) for the patients who received this device.
A randomized trial called INOVATE HF is now underway in the U.S. and Canada comparing CardioFit plus optimal medical care to medical care alone. Outcomes include heart-failure hospitalization and all-cause mortality.
The trial is unique in two ways: Patients with pre-existing ICDs may be enrolled, and each site must have a neurosurgeon as co-principal investigator.
“The CardioFit system requires the skill set of both specialists. If the device receives FDA approval, a neurosurgeon and electrophysiologist will have to work together on these patients,” says Dr. Cantillon, who is serving as principal site co-investigator with his neurosurgery colleague Andre Machado, MD. Randall Starling, MD, head of the Section of Heart Failure, is on the steering committee for North America.
Data from clinical trials of the WATCHMAN left atrial appendage (LAA) occlusion device are now being reviewed by the FDA. If the device is approved, “it will change practice by making it possible to wall off the LAA in a catheter-based procedure to reduce the risk of stroke,” Dr. Cantillon explains.
Advertisement
Cleveland Clinic was one of 65 centers that participated in the PROTECT AF trial. When compared with warfarin, the WATCHMAN reduced the rate of stroke 40 percent, cardiovascular death 60 percent and all-cause mortality 35 percent.
The Contact Force ablation catheter provides better, more consistent contact with tissue during ablation for atrial fibrillation, thereby producing greater freedom from arrhythmias. Its pressure sensor measures and displays contact force in real time, allowing the operator to avoid excess pressure that would increase the risk of tissue perforation. Cleveland Clinic was one of 22 centers participating in studies of the device that led to its FDA approval.
“I was not an investigator, but I became a fast adopter of this technology and now use it for almost all complex ablations,” says Dr. Cantillon.
Like pacemaker leads, ICD leads cause more than their share of complications. While the public was shocked by lead failures and fractures that led to high-profile recalls, physicians were also concerned about endovascular infections requiring extraction and complications and deaths from lead extraction procedures requiring laser tools to remove damaged or infected leads ingrown in the body.
For these reasons, FDA approval of the S-ICD, a leadless, subcutaneous ICD, was welcome news. The device has no leads and is not vulnerable to transvenous lead failures. Because it is implanted in soft tissue, it can be removed easily and safely if needed.
The S-ICD does have drawbacks, however, in that it does not have pacing capability or offer anti-tachycardia pacing. It’s also currently a little larger than a traditional ICD.
Advertisement
“These are important limitations,” says Dr. Cantillon. “However, the S-ICD is an excellent option for some patients, such as those with heritable conditions that render them vulnerable to sudden death at a young age, like Brugada syndrome.”
Someday, this device might be combined with a leadless pacemaker to create a safer ICD that could maintain pacing capabilities.
“I can foresee that future ICD platforms may supply some degree of pacing support without using leads,” says Dr. Cantillon.
Advertisement
Advertisement

A sampling of outcome and volume data from our Heart & Vascular Institute
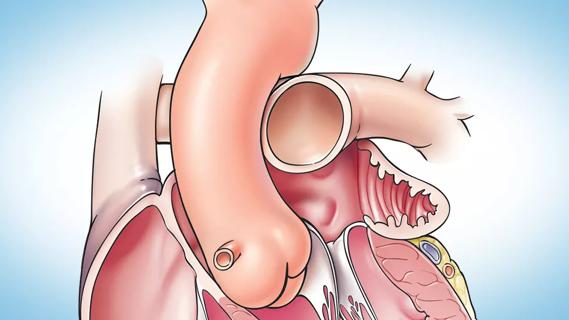
Concomitant AF ablation and LAA occlusion strongly endorsed during elective heart surgery
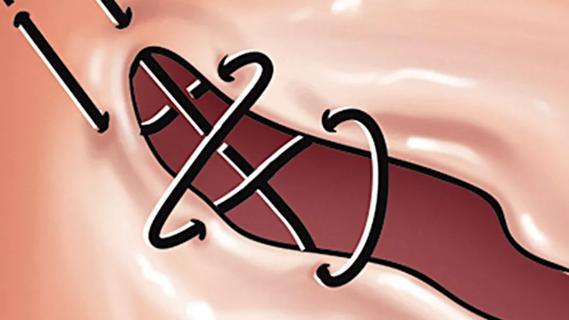
Large retrospective study supports its addition to BAV repair toolbox at expert centers
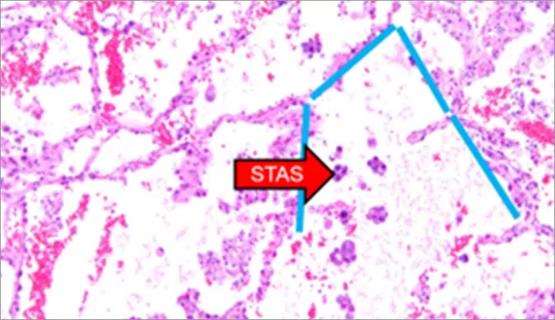
Young age, solid tumor, high uptake on PET and KRAS mutation signal risk, suggest need for lobectomy
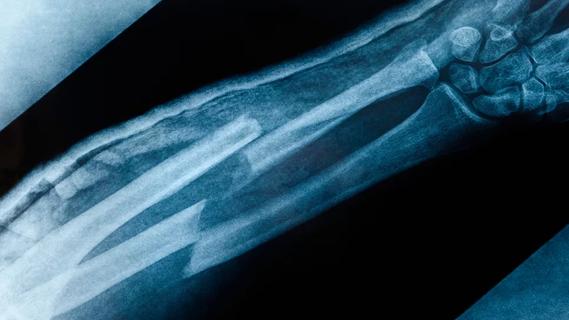
Surprise findings argue for caution about testosterone use in men at risk for fracture
Residual AR related to severe preoperative AR increases risk of progression, need for reoperation

Findings support emphasis on markers of frailty related to, but not dependent on, age
Provides option for patients previously deemed anatomically unsuitable