From prevention and pacing to stenting and surgery

Here are a few of the many clinical research trials underway in Cleveland Clinic’s Sydell and Arnold Miller Family Heart & Vascular Institute. For information on enrolling a patient in these or other trials, call 216.445.3608.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
PERIGON: Medtronic Pericardial Surgical Aortic Valve Replacement Pivotal Trial
Site PI: Joseph Sabik, MD (also national PI)
Description: Nonrandomized phase 3 trial evaluating the safety and effectiveness of the Medtronic Model 400 aortic valve bioprosthesis
Population: Patients requiring replacement of their native or prosthetic aortic valve (with or without concomitant procedures such as CABG surgery)
Sponsor: Medtronic Cardiovascular
Clinical Investigation of the Perceval S Sutureless Heart Valve
Site PI: Eric Roselli, MD
Description: Nonrandomized prospective study of the safety and effectiveness of the Perceval S heart valve to replace a diseased or dysfunctional native or prosthetic aortic valve
Population: Patients scheduled to undergo planned aortic valve replacement
Sponsor: Sorin Group USA
eMESH I: Study of External Saphenous Vein Support Using eSVS® Mesh in CABG Surgery
Site PI: Joseph Sabik, MD
Description: Randomized prospective study to assess the feasibility, initial safety and performance of the eSVS Mesh as an external vein support device for use over saphenous vein grafts (SVGs) during CABG surgery. Each enrollee receives an SVG without the mesh (control) and an SVG with the mesh (treatment).
Population: Patients requiring CABG surgery with SVGs to the right coronary artery system and the left circumflex artery system, with ≥ 70 percent stenosis in each system
Sponsor: Kips Bay Medical
LEADLESS II IDE: Safety and Effectiveness Trial for the Nanostim™ Leadless Pacemaker
Site PI: Daniel Cantillon, MD
Description: Phase 3 study of the safety and effectiveness of the Nanostim leadless pacemaker
Population: Patients with indications for a VVI pacemaker
Sponsor: St. Jude Medical
Advertisement
Micra Transcatheter Pacing Study
Site PI: Bruce Wilkoff, MD
Description: Phase 3 study to evaluate the safety and efficacy of the leadless Micra™ Transcatheter Pacing System and assess its long-term performance
Population: Patients with a class I or II indication for implantation of a single-chamber ventricular pacemaker
Sponsor: Medtronic Cardiac Rhythm Disease Management
reMARQable: nMARQ™ Pulmonary Vein Isolation System for the Treatment of Paroxysmal Atrial Fibrillation
Site PI: Bruce Lindsay, MD
Description: Randomized phase 3 trial evaluating the safety and effectiveness of the nMARQ Catheter System vs. ThermoCool® Navigation Catheters in treating drug-refractory symptomatic paroxysmal atrial fibrillation (AF)
Population: Patients with symptomatic paroxysmal AF who have had at least one AF episode in the prior year and failure of at least one antiarrhythmic drug
Sponsor: Biosense Webster
FIGHT: Functional Impact of GLP-1 for Heart Failure Treatment
Site PI: Wilson Tang, MD
Description: Randomized, double-blind phase 2 trial testing whether treatment with a subcutaneously delivered GLP-1 agonist in the period after acute heart failure syndrome discharge is associated with greater clinical stability (measured by a composite clinical endpoint) at six months compared with placebo
Population: Patients recently hospitalized for heart failure with an LVEF < 40 percent Sponsors: Duke University and NHLBI
GUIDE-IT: Guiding Evidence Based Therapy Using Biomarker Intensified Treatment
Site PI: Wilson Tang, MD
Description: Randomized open-label trial to determine the efficacy of a strategy of biomarker-guided therapy compared with usual care in high-risk patients with left ventricular systolic dysfunction
Population: Heart failure patients with a recent LVEF < 40 percent and recent hospitalization or ED visit for heart failure
Sponsors: Duke University and NHLBI
Advertisement
NEAT: Nitrate’s Effect on Activity Tolerance in Heart Failure with Preserved Ejection Fraction
Site PI: Wilson Tang, MD
Description: Randomized, double-blind, placebo-controlled crossover study assessing the effect of isosorbide mononitrate with upward dose titration on activity tolerance as assessed by accelerometry
Population: Heart failure patients with preserved ejection fraction
Sponsors: Duke University and NHLBI
HCMR: Novel Markers of Prognosis in Hypertrophic Cardiomyopathy
Site PI: Milind Desai, MD
Description: Patient registry study to develop a predictive model of cardiovascular outcomes in hypertrophic cardiomyopathy (HCM) by using data mining to identify demographic, clinical and novel cardiovascular magnetic resonance, genetic and biomarker variables associated with outcomes
Population: Adults up to age 65 with an established diagnosis of HCM defined as unexplained left ventricular hypertrophy
Sponsors: University of Virginia and NHLBI
VANISH: Valsartan for Attenuating Disease Evolution in Early Sarcomeric HCM
Site PI: Harry Lever, MD
Description: Randomized, double-blind, placebo-controlled phase 2 trial assessing the safety and efficacy of valsartan in attenuating disease evolution in early hypertrophic cardiomyopathy (HCM)
Population: Patients with a pathogenic or likely pathogenic HCM sarcomere mutation
Sponsors: New England Research Institutes and NHLBI
ABSORB IV: A Clinical Evaluation of Absorb™ BVS, the Everolimus Eluting Bioresorbable Vascular Scaffold, in the Treatment of Subjects with De Novo Native Coronary Artery Lesions
Site PI: Russell Raymond, DO
Description: Randomized, controlled, single-blind evaluation of the Absorb BVS everolimus-eluting bioresorbable vascular scaffold compared with XIENCE stents for the treatment of denovo native coronary artery lesions
Population: Patients with de novo native coronary artery lesions
Sponsor: Abbott Vascular
Advertisement
SALUS: The Direct Flow Medical Transcatheter Aortic Valve Replacement System: A U.S. Pivotal Trial
Site PI: Samir Kapadia, MD
Description: Open-label study to assess the safety and effectiveness of the Direct Flow Medical aortic valve system for patients with severe aortic stenosis who are not well enough to undergo surgical repair
Population: Patients with severe symptomatic aortic stenosis at high risk or deemed not suitable for surgery following evaluation by a cardiac surgeon
Sponsor: Direct Flow Medical
GAUSS-3: Trial of Patients with Statin Intolerance Randomized to PCSK9 vs. Zetia
Site PI: Michael Rocco, MD
Description: Randomized, double-blind, controlled phase 3 study of AMG 145 (evolocumab) in statin-intolerant individuals with dyslipidemia to assess side effects and efficacy in lowering LDL cholesterol and increasing HDL cholesterol
Population: Patients ages 18 to 80 who are not at goal LDL cholesterol, have fasting triglycerides ≤ 400 mg/dL, have a history of intolerance to at least two statins, and currently are not on a statin or are on a stable low dose
Sponsor: Amgen
GRADY: Gut Flora Metabolite Reduction After Dietary Intervention
Site PIs: Wilson Tang, MD, and Stanley Hazen, MD, PhD
Description: Randomized, open-label phase 1/2 study investigating the ability of dietary intervention to modulate TMAO levels with and without TMAO levels being provided to subjects for guidance
Population: Patients with elevated TMAO metabolizers (> 5 μM), based on a screening test, who are willing to follow a modified Mediterranean diet for 12 weeks
Sponsor: Cleveland Clinic
Advertisement
STRENGTH: Outcomes Study to Assess Statin Residual Risk Reduction with Epanova® in High CV Risk Patients with Hypertriglyceridemia
Site PI: Michael Rocco, MD
Description: Double-blind, controlled, parallel-group phase 3 study randomizing 13,000 patients to either Epanova (omega-3 carboxylic acids) plus statin therapy or corn oil plus statin therapy for three to five years to assess for major adverse cardiovascular events
Population: Eligible adults considered to be at high risk for atherosclerotic cardiovascular disease
Sponsor: AstraZeneca, with Cleveland Clinic as collaborator
ATTRACT: Acute Venous Thrombosis: Thrombus Removal with Adjunctive Catheter-Directed Thrombolysis
Site PI: Heather Gornik, MD
Description: Randomized open-label study to determine whether pharmacomechanical catheter-directed thrombolysis prevents post-thrombotic syndrome and improves quality of life in patients with DVT compared with optimal standard DVT therapy alone
Population: Patients ages 16 through 75 with symptomatic proximal DVT involving the iliac, common femoral and/or femoral veins
Sponsor: Washington University School of Medicine
PRYME: Prospective Registry of Young Women with MI: Evaluating the Prevalence and Long-term Impact of Nonatherosclerotic CAD
Site PI: Heather Gornik, MD
Description: Prospective observational registry study to evaluate the long-term outcome of young women with nonatherosclerotic coronary artery disease (CAD) relative to those with atherosclerotic CAD over five years of follow up
Population: Women 55 or younger with a troponin-positive acute coronary syndrome who have a coronary angiogram
Sponsors: Cardiology Research UBC and University of British Columbia
EVAS IDE: Safety and Effectiveness Study of Endovascular Abdominal Aortic Aneurysm Repair Using the Nellix® System
Site PI: Daniel Clair, MD
Description: Prospective single-arm study assessing the safety and effectiveness of the investigational Nellix System for the endovascular repair of infrarenal abdominal aortic aneurysms
Population: Patients with abdominal aortic aneurysms
Sponsor: Endologix
Lutonix® Drug Coated Balloon Versus Standard Balloon Angioplasty for Treatment of Below-the-Knee Arteries
Site PI: Sean Lyden, MD
Description: Randomized single-blind study comparing the Lutonix Drug Coated Balloon with standard balloon angioplasty for safety and efficacy in the treatment of stenosis or occlusion of native below-the-knee arteries
Population: Patients with stenosis or occlusion of native below-the-knee arteries
Sponsor: C.R. Bard
Advertisement

A sampling of outcome and volume data from our Heart & Vascular Institute
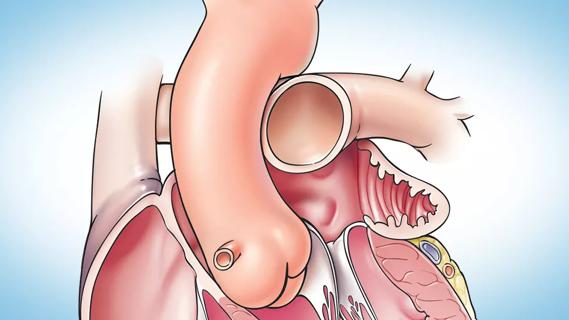
Concomitant AF ablation and LAA occlusion strongly endorsed during elective heart surgery
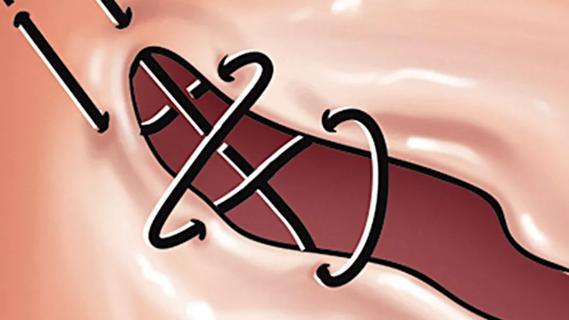
Large retrospective study supports its addition to BAV repair toolbox at expert centers
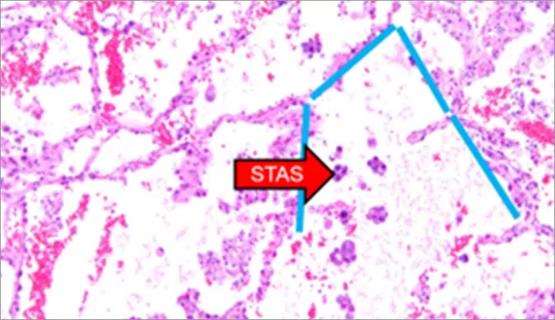
Young age, solid tumor, high uptake on PET and KRAS mutation signal risk, suggest need for lobectomy
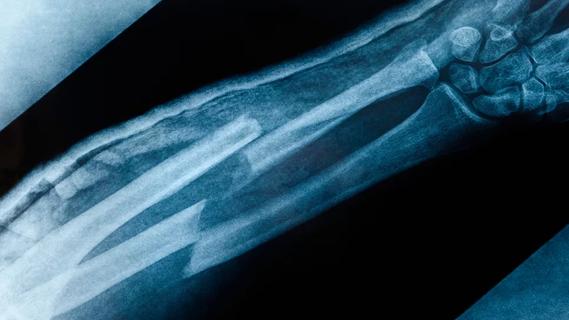
Surprise findings argue for caution about testosterone use in men at risk for fracture
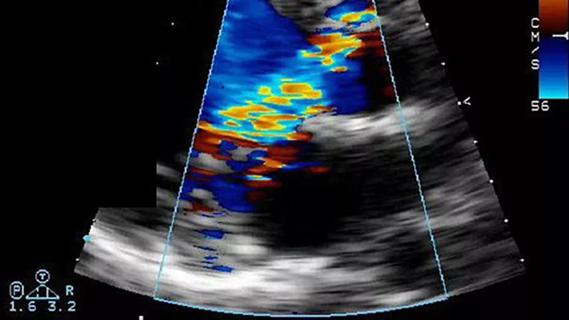
Residual AR related to severe preoperative AR increases risk of progression, need for reoperation

Findings support emphasis on markers of frailty related to, but not dependent on, age
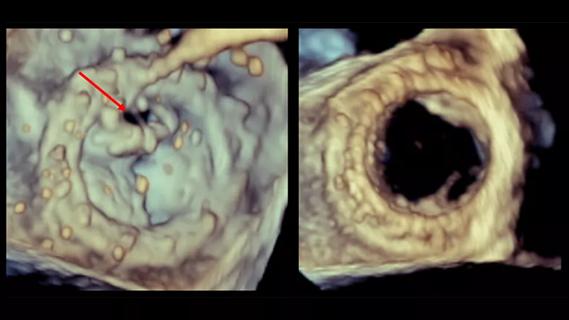
Provides option for patients previously deemed anatomically unsuitable