Lingering uncertainties may eventually give way to global registry

Advances in cardiac and obstetrical care are enabling most women with congenital heart disease to successfully conceive and bear children, as underscored by a new scientific statement from the American Heart Association (AHA) reported here recently. But evidence-based information on the effect of heart medications during pregnancy is still incomplete and being revised as new drugs come to market with limited research data on their effects on the pregnant mother and the fetus.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
The section in the new AHA scientific statement devoted to the use of common heart medications during pregnancy in patients with congenital heart disease is an updated source to which clinicians can turn for advice. “The authors address routine issues faced by physicians who take care of women with heart disease,” says cardiologist David Majdalany, MD, Director of Cleveland Clinic’s Adult Congenital Heart Disease Center.
Yet the AHA statement also illustrates the incomplete knowledge about the use of heart medications during pregnancy: The section is less than one page long, though it’s supplemented by a handy three-page table outlining pregnancy-related considerations around the use of antihypertensives, diuretics, anticoagulants, inotropes/vasopressors and antiarrhythmics. All but one of the dozens of listed agents are classified B, C or D by the FDA, indicating a dearth of information from clinical trials or evidence of human fetal risk.
“This is part of the challenge of our practice,” Dr. Majdalany observes.
A central issue is how drug metabolism is altered during pregnancy. “Changes in metabolism with most heart medications have not been thoroughly studied,” Dr. Majdalany says, noting that dose adjustments may be needed for some medications during pregnancy.
The increase in blood volume that occurs during pregnancy affects blood flow to the kidneys and liver, the primary metabolic pathways for most heart drugs. “You need to be cognizant of drug clearance rates and how they affect dose and monitoring,” he adds.
Advertisement
Although a majority of heart drugs carry a C classification from the FDA due to insufficient data, Dr. Majdalany deems some agents “C+” or “B–” because they tend to be safer than others. “Drugs like beta-blockers, diuretics and most calcium channel blockers are relatively safe,” he says. “The benefits may outweigh the risks, especially when we’re treating arrhythmias during pregnancy.”
All efforts should be made to resolve cardiomyopathy prior to conception, he notes, since a heart that has recovered function will be better able to handle the stress of pregnancy.
ACE inhibitors and angiotensin receptor blockers — the mainstay of cardiomyopathy treatment — are contraindicated during pregnancy. When an alternative to these medications is needed, Dr. Majdalany turns to vasodilators. “I tend to use hydralazine, sometimes in combination with a nitrate,” he says.
The fact that pregnancy is a hypercoagulable state, with increased risk of venous thrombosis, poses particular concern for women who have had a prior embolism, mechanical heart valves or atrial arrhythmias. When these women become pregnant, finding an anticoagulation regimen that’s safe for mother and fetus can be challenging.
Warfarin carries an FDA classification of X, due to its known teratogenicity during the first trimester. “Low-dose warfarin is used in Europe, but it’s difficult to achieve a therapeutic range with that dose,” Dr. Majdalany notes.
Unfractionated heparin is an alternative, but its intravenous formulation is inconvenient. Twice-daily injections of the low-molecular-weight heparin enoxaparin may be an option, as it doesn’t cross the placenta. The drawback is that heparin use in patients with mechanical valves has a higher thrombosis risk relative to warfarin.
Advertisement
And novel anticoagulants such as rivaroxaban (Xarelto®) and dabigatran (Pradaxa®) have not been adequately studied as alternatives in pregnancy.
Dr. Majdalany’s solution is to split the anticoagulation regimen between enoxaparin and warfarin. “I use enoxaparin during the first trimester and follow anti-factor Xa levels closely to ensure the dosage is therapeutic,” he says. “By the second trimester, the potential effects of warfarin are no longer a concern, so I switch. Closer to delivery, I switch back to enoxaparin or unfractionated heparin. There is no perfect regimen.”
While it’s unethical to randomize pregnant patients in a clinical trial, the need for evidence-based treatments for this population is intense. Cleveland Clinic hopes its participation in the international Registry of Pregnancy and Cardiac Disease (ROPAC) may help provide badly needed data.
ROPAC provides a central database for pooling data on pregnant patients with cardiovascular disease. Participating centers complete a detailed form on every patient, noting diagnosis, treatment plan, complications, mode of delivery, outcomes and more. The goal is better understanding of how each form of heart disease and its treatments are impacted by pregnancy.
“The more patients enrolled, the more the data will be applicable,” Dr. Majdalany explains. “We are trying to make gray zones black or white.”
Until answers emerge, outcomes can be optimized by ensuring high-risk patients are treated by a multidisciplinary team, he says. “For now, nothing beats the combined experience and opinions of a well-organized team that works well together.”
Advertisement
Advertisement

A sampling of outcome and volume data from our Heart & Vascular Institute
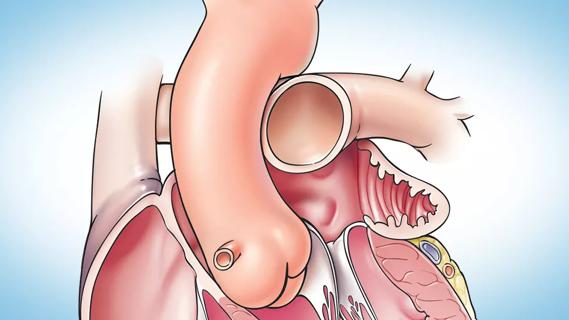
Concomitant AF ablation and LAA occlusion strongly endorsed during elective heart surgery
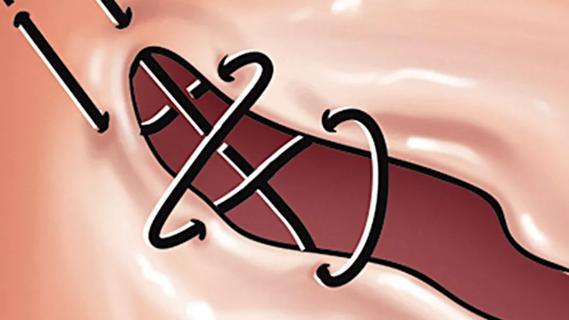
Large retrospective study supports its addition to BAV repair toolbox at expert centers
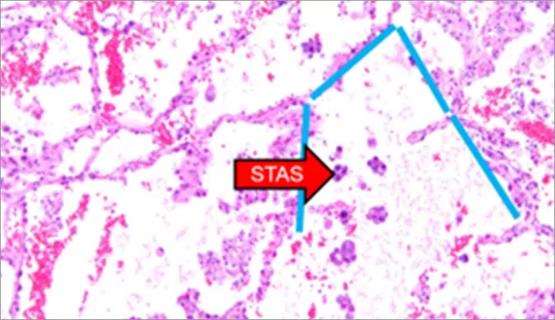
Young age, solid tumor, high uptake on PET and KRAS mutation signal risk, suggest need for lobectomy
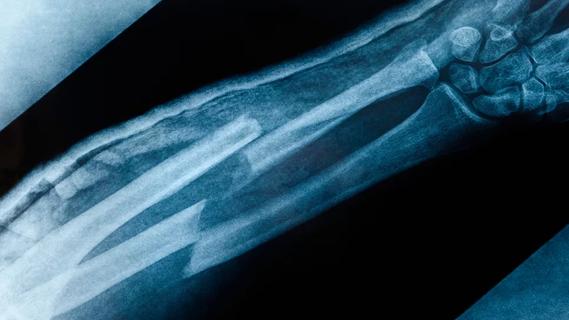
Surprise findings argue for caution about testosterone use in men at risk for fracture
Residual AR related to severe preoperative AR increases risk of progression, need for reoperation

Findings support emphasis on markers of frailty related to, but not dependent on, age
Provides option for patients previously deemed anatomically unsuitable