Enrollment continues in ABSORB trial comparing new scaffold to metallic drug-eluting stents

Enrollment in Abbott’s ABSORB III™ clinical trial, launched in 2013, continues apace, with the goal of enrolling approximately 2,250 patients in the U.S. The randomized controlled trial is designed to compare the performance of Abbott’s drug-eluting Absorb™ Bioabsorbable Vascular Scaffold (BVS) device with the company’s XIENCE™ family of drug-eluting stents.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
Absorb is a second-generation drug-eluting, fully bioabsorbable scaffold constructed from polylactide, a naturally dissolvable material commonly used in sutures. Similar to a metallic drug-eluting stent, the BVS works by opening a clogged vessel and restoring blood flow to the heart. While still the standard of care for coronary lesions, metallic drug-eluting stents leave behind metal architecture and residual polymer, which cause complications in some patients.
With an Absorb BVS, all that remains after the scaffold dissolves are four tiny metallic markers. The markers’ sole purpose is to help physicians position the scaffold.
The Absorb BVS delivers everolimus, the same antiproliferative agent that Abbott uses in its XIENCE stents.
Stephen Ellis, MD, Section Head of Interventional Cardiology at Cleveland Clinic and Co-primary Investigator on the ABSORB III trial, says the study will address issues that are experienced in patients who receive current state-of-the-art drug-eluting stents (DES).
“The long-term presence of a polymer in an artery is prothrombotic,” Dr. Ellis says. “Current DES contain a very durable polymer.” The next iteration of these scaffolds, he says, will hopefully address this and other concerns.
Dr. Ellis says that patients with DES experience a stent thrombosis rate of 0.3 to 0.8 percent per year over five years, often leading to heart attack or death. The totally bioabsorbable product seeks to improve on that rate.
The ultimate goal, Dr. Ellis adds, is to open a blocked vessel yet leave very little behind so that the opened vessel regains more natural motion after the scaffold has fully dissolved, something not achieved by current metal scaffolds.
Advertisement
In human studies performed overseas, the Absorb BVS loses its structural integrity over the course of the first year, and dissolves completely in two to three years.
A dissolving polymer causes less inflammation in the vessel, which allows for more natural vasomotion when the patient participates in normal activities such as exercise. But the perfect balance must be struck: The scaffold needs to remain largely intact long enough to allow the everolimus to do its work and then allow as much of the natural vessel function to return as possible.
The first-generation scaffold dissolved too quickly and was withdrawn from evaluation. The second-generation Absorb scaffold uses a combination of polymers that addresses the problem of a bioabsorbable scaffold degrading too quickly while it also further improves on a restenosis rate that has decreased with the change from purely metal scaffolds to the current metal, polymer and drug devices.
“By providing a device that initially matches the performance of DES and then totally resorbs, we hope that the occasional long-term complications with standard stents will be eliminated,” says Dr. Ellis.
The study is enrolling patients at 180 sites across the country who have already been determined to be good candidates for stenting. Enrollees must not have more than two native coronary artery lesions in separate epicardial vessels, neither of which can be near a branch. Patients cannot have a known allergy to the polymers from which the scaffold is constructed. The limit for the scaffold length is 24 mm, and the area for treatment must be a simple lesion. Appropriate patients will be randomized 2-to-1 for scaffold vs. current DES
Advertisement
Target lesion failure (death, heart attack or need to treat the site again) at one year is the primary endpoint of the study; a subset of patients will be evaluated for other endpoints, such as vasomotion. Several five-year outcome measures will indicate how well the vessel remains open.
The estimated study completion date is March 2018, with primary outcome measure data due to be collected by August 2015.
Absorb is available commercially in Europe, India, and parts of Latin America and Asia. In June 2013, a randomized controlled trial designed to enroll 400 patients was initiated in Japan.
Advertisement
Advertisement

A sampling of outcome and volume data from our Heart & Vascular Institute
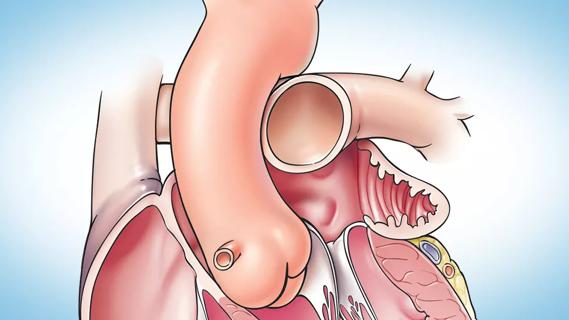
Concomitant AF ablation and LAA occlusion strongly endorsed during elective heart surgery
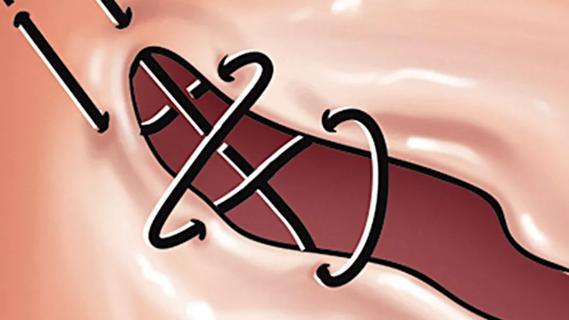
Large retrospective study supports its addition to BAV repair toolbox at expert centers
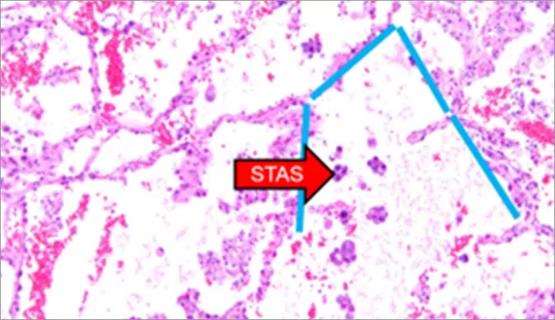
Young age, solid tumor, high uptake on PET and KRAS mutation signal risk, suggest need for lobectomy
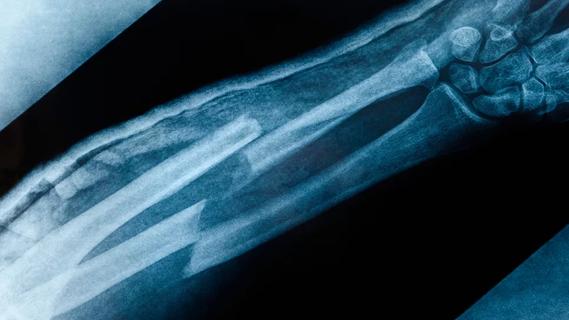
Surprise findings argue for caution about testosterone use in men at risk for fracture
Residual AR related to severe preoperative AR increases risk of progression, need for reoperation

Findings support emphasis on markers of frailty related to, but not dependent on, age
Provides option for patients previously deemed anatomically unsuitable