Device integrates wireless communication between leadless pacing and ICD therapy
An international trial of the world’s first leadless pacemaker-defibrillator system has been launched with implantation of the system in the study’s first two patients. The first implantations were done at Cleveland Clinic, which is leading the trial.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
Designed to treat sudden cardiac arrest without transvenous leads, the investigational system uses pacing stimulation to painlessly disrupt lethal fast rhythms such as ventricular tachycardia and provide emergency backup pacing for dangerously slow rhythms like asystole. If pacing fails to restore sinus rhythm, the system’s defibrillator component delivers a shock as a last resort.
The trial, known as MODULAR ATP, is evaluating the effectiveness and safety of the Modular Cardiac Rhythm Management (mCRM) Therapy System. Global principal investigator Daniel Cantillon, MD, is excited by how far rhythm management technology has come since 2014, when he implanted the world’s first leadless pacemaker.
“We’ve been working on this technology so long that the first cases feel like the end of a preclinical journey,” says Dr. Cantillon, who is a consultant for Boston Scientific, the manufacturer of the system and sponsor of MODULAR ATP. “We view this as an important solution for getting patients away from the long-term problems associated with transvenous implantable cardioverter-defibrillators [ICDs].”
The mCRM Modular Therapy System combines the EMPOWER™ Modular Pacing System and the EMBLEM™ MRI Subcutaneous ICD.
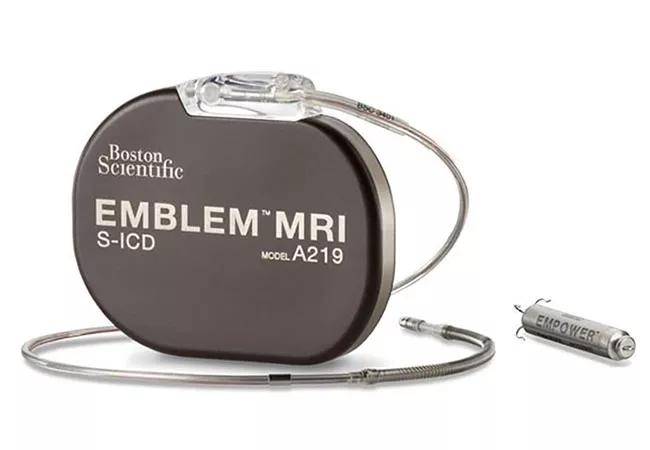
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/24133cca-4957-40da-96ce-e8ecb9b7dfbe/21-HVI-2577808_Original-photo_650x450_jpg)
Image courtesy of Boston Scientific ©2021. The mCRM Therapy System is an investigational device and limited by U.S. law to investigational use only. It is not available for sale.
The pacemaker component uses a flexible, telescoping catheter delivery system that facilitates its delivery and attachment to the myocardium, making it potentially safer. The absence of leads avoids common problems that affect approximately one in six patients with traditional pacemaker-defibrillators within three months of implantation, including lead fractures, dislodgements and venous occlusive disease.
Advertisement
What’s most notable, Dr. Cantillon says, is that the pacemaker communicates with the ICD component. When the subcutaneous ICD detects a lethal arrhythmia, it analyzes the rhythm while charging its battery in preparation for delivering a life-saving shock. In those crucial seconds, it sends a coded message through the patient’s body to the pacemaker, instructing it to produce a specific sequence of preprogrammed electrical pulses.
“Prior studies suggest this can successfully terminate up to 75% of cases of monomorphic ventricular tachycardia, which is by far the most common lethal arrhythmia,” Dr. Cantillon notes.
If it succeeds, the ICD stands down and diffuses its charge, and the patient remains unaware that the intervention occurred. If the pacing fails, the ICD immediately delivers the rescue shock.
MODULAR ATP will enroll up to 300 patients at 50 centers in the U.S., Canada, Europe and Asia.
Dr. Cantillon successfully implanted the new system in the trial’s first two patients. Both patients are typical of those who will be enrolled in MODULAR ATP: they have heart failure and received shocks from transvenous ICDs that were painful and resulted in psychological trauma.
The single-arm, open-label trial will evaluate the effectiveness and safety of the wireless system.
Although the ability of the two component devices to communicate is tested in the procedural room at the time of implantation, study participants will undergo positional testing to noninvasively verify that signals are being sent and received.
Advertisement
System- and procedure-related complications will be assessed through six and 12 months. How often the system delivers rescue therapy without delivering shocks will also be examined.
Dr. Cantillon expects preliminary results to be available in 12 to 24 months, but he emphasizes that safety will not be compromised in the interest of speed.
“This system must be handled by extremely experienced operators and implanted in the right patients,” he says. “If we take the time to do it right and get good data to submit to the FDA, it may be reasonable to think this system could be commercially available in two to three years.”
Beyond this trial, Dr. Cantillon is energized by the promise that modular wireless technology holds for the millions of patients with heart failure worldwide who need ICDs to protect them from sudden cardiac death. Whereas some don’t need pacing support and can receive the subcutaneous ICD alone, others need both the ICD and a pacemaker. The modular aspect of emerging technology means that these elements can be added at different times.
“Once you demonstrate the feasibility of using the patient’s own body to send and receive signals, you can branch out,” Dr. Cantillon explains. “We expect future technological iterations to allow coordination of multichamber leadless pacing with the subcutaneous ICD to serve an even greater number of patients requiring pacing support for exercise and physical activity.”
“This is a great development in device therapy for our patients that will ultimately lead to better clinical results with less long-term risk,” observes Oussama Wazni, MD, MBA, Section Head of Cardiac Electrophysiology and Pacing at Cleveland Clinic. “Dr. Cantillon has been a leader in this field and will continue to collaborate in pioneering this technology.”
Advertisement
Advertisement
A sampling of outcome and volume data from our Heart & Vascular Institute
Concomitant AF ablation and LAA occlusion strongly endorsed during elective heart surgery
Large retrospective study supports its addition to BAV repair toolbox at expert centers
Young age, solid tumor, high uptake on PET and KRAS mutation signal risk, suggest need for lobectomy
Surprise findings argue for caution about testosterone use in men at risk for fracture
Residual AR related to severe preoperative AR increases risk of progression, need for reoperation
Findings support emphasis on markers of frailty related to, but not dependent on, age
Provides option for patients previously deemed anatomically unsuitable