Training, surgical planning are possible uses
By Jihad H. Kaouk, MD and Peter Caputo, MD
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy

Jihad H. Kaouk, MD
Three-dimensional (3-D) printing is a new technology that is rapidly being incorporated into the practice of medicine.
The technology involves robotically depositing successive layers of material — plastic, metal or even biological tissue — under computer control to form objects of virtually unlimited shape or geometry. 3-D printing is proving useful in the arena of regenerative medicine, with the manufacture of customized surgical implants, prosthetics and medical devices. In the near future, 3-D bio-printing with living tissue may allow the production of replacement organs and body parts.
At Cleveland Clinic’s Glickman Urological & Kidney Institute, we strive to find innovative methods to improve patient care and to introduce new information to physicians-in-training and patients. Currently we are investigating 3-D printing for training and educational purposes.
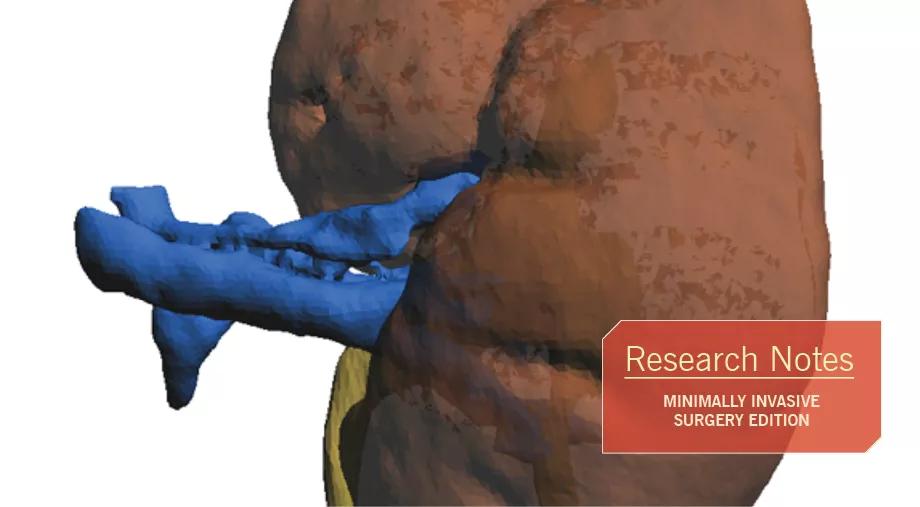
Cross-sectional imaging allows for accurate 3-D rendering of individual patient anatomy. From this rendering we are able to print a 3-D structure that precisely replicates the unique renal anatomy.
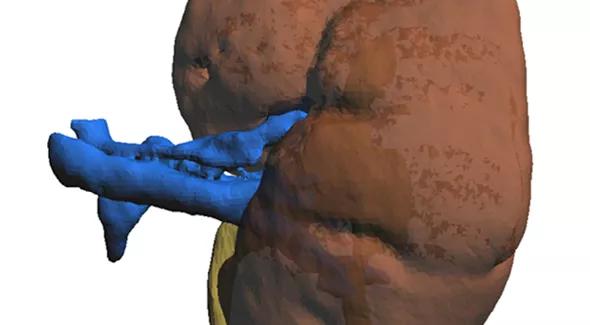
Figure 1. A high-resolution reconstruction image based on 3 mm crosscuts from a patient’s kidney CT scan. The reconstructed image allows for detailed identification of kidney vasculature, collecting system and parenchyma, including tumor characteristics. A high-resolution image is crucial for 3-D printing.
We have found that by using imaging-based 3-D kidney models as an educational and visualization aid, medical students and resident physicians are better able to characterize a particular patient’s renal tumor.
Advertisement
This visualization benefit extends to patients, too. Patients with newly discovered renal masses can hold and examine a 3-D rendering of their kidney and tumor, helping us educate them about their condition and further engage them in their care. Studies have shown that by improving patients’ health literacy, we improve their ability to participate in important healthcare decisions, which can lead to better outcomes.
Application of these 3-D kidney models to patient-specific surgical scenarios may also benefit our surgical trainees, with the goal of shortening the learning curve for difficult surgical procedures. Patient-specific 3-D kidney models utilized for preoperative planning and even surgical simulation may enable a trainee to obtain fewer positive margins, shorten ischemic times and preserve more viable kidney parenchyma.
The use of 3-D renal models for surgical simulation may help train the next generation of surgeons. The combination of a 3-D model and a robotic surgical system could provide a surgical simulation that very closely mimics real-life surgical scenarios, allowing surgical residents and novice surgeons the opportunity for hands-on robotic system experience before ever entering the operating room.
Additionally, 3-D models are being used in the development of automated surgical approaches. In this scenario, a skilled surgeon using a patient-specific 3-D renal model controls the robotic system to remove a tumor from the surrounding normal kidney.
The surgeon repeats this procedure several times on identical 3-D models while the robotic system analyzes and records each of the surgeon’s movements. The surgeon and robotic system are then able to select the most successful surgical movements specific to the patient’s anatomy and store them for future use.
Advertisement
Applying that stored information after the robotic surgical system has been spatially oriented in a live surgery should allow the completion of a complex surgical procedure in a fraction of the time required for a conventional surgery. Although the implementation of automated surgical technology is many generations away, the aim is to provide high-quality, patient-specific automated surgery that will translate to better outcomes.
Dr. Kaouk is Director of Cleveland Clinic Glickman Urological & Kidney Institute’s Center for Robotic and Laparoscopic Surgery and is the Urological & Kidney Institute’s Vice Chair for Surgical Innovations. He is a Professor of Surgery at Cleveland Clinic Lerner College of Medicine.
Dr. Caputo is a fellow in Cleveland Clinic Glickman Urological & Kidney Institute’s Department of Urology.
Advertisement
Advertisement

Clinicians should individualize dosing practices based on patient risk factors and preferences
Pioneering and refining the approach in pyeloplasty, nephrectomy and more
Fully-automated process uses preop CT, baseline GFR to estimate post-nephrectomy renal function
Could mean earlier treatment, but also could have negative effects

Unlike earlier pills, new drugs do not cause liver toxicity

Male factors play a role in about half of all infertility cases, yet men often are not evaluated
Surgeons choreograph nearly simultaneous procedures, sharing one robot between two patients

Identifying barriers in the renal genetic assessment of Black patients