Insights from the PI of the SENTINEL trial
One essential question in the wake of the FDA’s approval of the Sentinel® Cerebral Protection System earlier this month is this: Which patients should get the device?
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
As discussed in a previous Consult QD post on the Sentinel approval, the formal indication is “for use as an embolic protection device to capture and remove thrombus/debris while performing transcatheter aortic valve replacement [TAVR] procedures,” but that leaves patient selection open to clinical judgment.
In the 90-second video below, Cleveland Clinic interventional cardiologist Samir Kapadia, MD, uses his experience as lead investigator of the pivotal SENTINEL trial, which formed the basis of the device’s approval, to offer some perspective on this question. As one of the high-volume TAVR centers of excellence in which the device has initially been launched — and the only site in Ohio and its five surrounding states — Cleveland Clinic has already been putting these patient-selection principles into practice. (For a longer segment in which Dr. Kapadia discusses the Sentinel approval more broadly, see the “Cerebral Protection in TAVR” video here.)
Advertisement
Advertisement

A sampling of outcome and volume data from our Heart & Vascular Institute
Concomitant AF ablation and LAA occlusion strongly endorsed during elective heart surgery
Large retrospective study supports its addition to BAV repair toolbox at expert centers
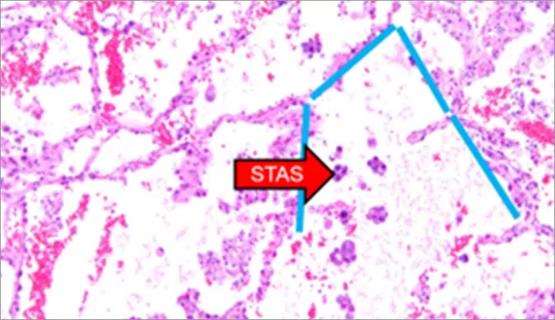
Young age, solid tumor, high uptake on PET and KRAS mutation signal risk, suggest need for lobectomy
Surprise findings argue for caution about testosterone use in men at risk for fracture
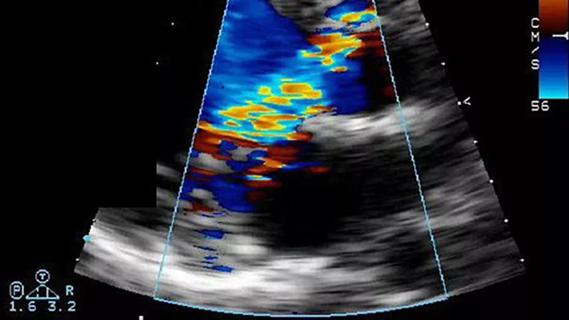
Residual AR related to severe preoperative AR increases risk of progression, need for reoperation

Findings support emphasis on markers of frailty related to, but not dependent on, age
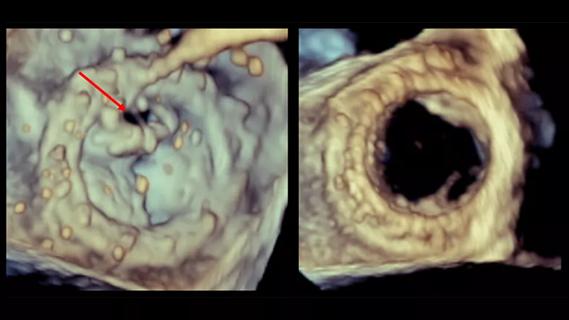
Provides option for patients previously deemed anatomically unsuitable