Insights from fMRI study may aid treatment monitoring
By Dietmar Cordes, PhD; Xiaowei Zhuang, MS; Zhengshi Yang, MS; Christopher Bird, BA; and Tim Curran, PhD
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
The critical role of the hippocampus and the nearby medial temporal lobe (MTL) cortex in learning and memory is well documented, especially from neuropsychological studies of anterograde amnesia that results from damage to these areas.1,2 Although high-resolution fMRI provides valuable insight into specific MTL subregions involved in episodic memory, few studies have been completed, and the precise nature of the function of hippocampal subfields and the adjacent MTL cortical subregions remains unclear.
Animal models have indicated an input/output transfer function for neural activity in hippocampal subfields, which is different for memory processes involving pattern separation processes (Δ input < Δ output) as opposed to pattern completion processes (Δ input > Δ output). In this model, neurons in the cornu ammonis area 1 region (CA1) respond linearly to the change of input, whereas neurons in the dentate gyrus (DG) are more sensitive and respond nonlinearly to small increments in the variation of input (Figure 1, left panel).3-5
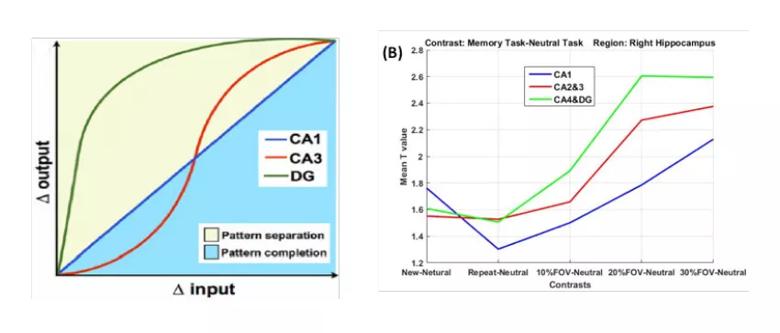
Figure 1. Pattern separation and pattern completion in the CA1, CA3 and DG regions. (Left) Animal model showing input/output transfer function, linear in CA1, nonlinear in CA3 and DG. Δ input = increments of change in sensory input; Δ output = differences in target output. Reprinted from ref. 5 (Yassa and Stark, Trends in Neurosciences, ©2011, with permission from Elsevier). (Right) Mean activation t-value in each of the hippocampal subfields from the current study: right CA1 (blue), right CA2&3 (red) and CA4&DG (green) as a function of lure difference (equivalent to Δ input).
Advertisement
Cleveland Clinic Lou Ruvo Center for Brain Health has conducted an fMRI study to investigate this input/output transfer relationship in human hippocampal subfields (mainly CA1, CA3 and DG) during a spatial memory task using a multivariate analysis approach. The innovation of this study stems primarily from the advanced data analysis techniques we have used to obtain more specific activation maps than standard univariate methods are able to achieve. We adapted a locally constrained CCA (cCCA) multivariate analysis technique6,7 that uses a spatially adaptive filter kernel. We recently improved this technique by using dominance constraints to significantly increase the specificity of the analysis,8 thereby reducing artifacts that are associated with a spatially increased smoothing kernel.
The technical innovation of our cCCA research is that this method can be transformed into a mathematically equivalent form of a constrained, multivariate multiple regression problem for which any linear contrast of interest can be readily computed.
In a current research project funded by the National Institutes of Health, we applied our multivariate analysis technique to high-resolution (1.6 × 1.6 × 2.0 mm) fMRI data obtained in subregions of the human hippocampus and segmented using a 0.8-mm isotropic three-dimensional T1 structural image to obtain activation in hippocampal subfields (Figure 2, left panel) at 3 tesla. During this study, a repetition suppression spatial memory task was performed to examine differences in pattern separation and completion tendencies of hippocampal subfields. The task design involved viewing pairs of visual objects presented for a first time, repetitions of the same objects, or lures that were visually similar to the first-time objects. Three types of lures were presented, which differed from the original objects by a separation distance of 10 percent field of view (FOV), 20 percent FOV or 30 percent FOV. Additionally, a distraction task occurred occasionally, requiring subjects to perform simple addition (Figure 2, right panel).
Advertisement
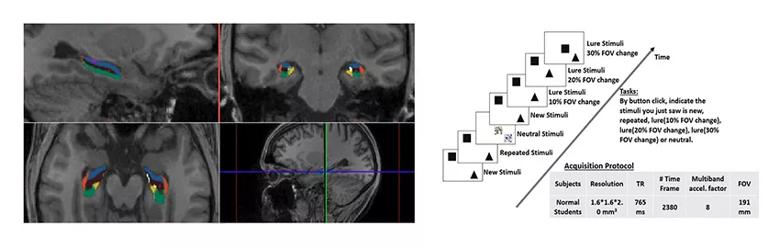
Figure 2. Experimental design. (Left) Hippocampal segmentation on T1-weighted MRI, acquired with Freesurfer. Color key: red = CA1, blue = CA2&3, brown = CA4&DG (other subregions: purple = fimbria, white = hippocampal fissure, yellow = presubiculum, green = subiculum). (Right) High-resolution functional MRI experimental task design and imaging protocol. Tasks include button click in response to new stimuli, repeated stimuli, lure stimuli with small, medium and large changes (Δ input), and distraction stimuli.
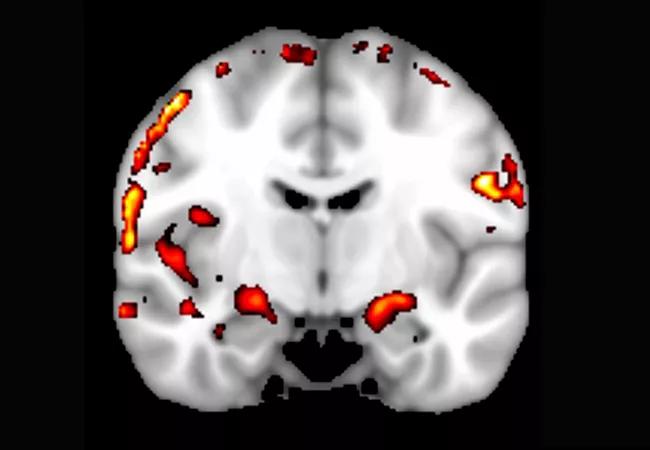
We were able to obtain bilateral activations in the hippocampus for all lures (when compared with the distraction task; P < .001) (see Figure 3, whole brain activation map for lure with the 20 percent FOV change). Figure 3 also shows that the memory activation was clearly localized to gray matter regions in the medial and superior temporal lobes, but not to the white matter, which is evidence that artifacts are minimal.
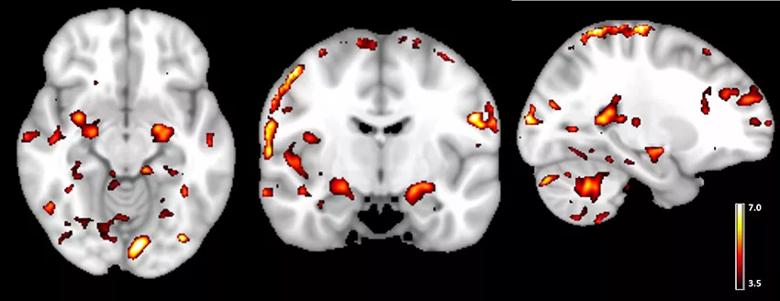
Figure 3. Whole brain activation during a memory-related task (t-statistic map for contrast: memory task – control task; threshold at t > 3.5, P < .001). Note that activations are mostly in the gray matter.
Furthermore, we obtained a similar input/output relationship for activations in the right CA1, CA3 and DG regions as separation distances in lure stimuli increased. As in the animal model, the DG is the most sensitive region in response to a small change in input, whereas all three subregions are highly activated with large differences in separation distances (Figure 1, right panel).
Advertisement
In conclusion, we were able to determine hippocampal subfield-specific memory activations as a function of the separation distance of the lures. We are planning to develop a simpler version of this spatial memory task with clinical applications that include, for example, monitoring treatment effects of human memory disorders.
Advertisement
Dr. Cordes is an associate staff member in the Cleveland Clinic Lou Ruvo Center for Brain Health in Las Vegas.
Dr. Curran is a professor in the Department of Psychology and Neuroscience at the University of Colorado, Boulder.
Advertisement

New study advances understanding of patient-defined goals

Testing options and therapies are expanding for this poorly understood sleep disorder
Real-world claims data and tissue culture studies set the stage for randomized clinical testing
Digital subtraction angiography remains central to assessment of ‘benign’ PMSAH
Cleveland Clinic neuromuscular specialist shares insights on AI in his field and beyond

Findings challenge dogma that microglia are exclusively destructive regardless of location in brain

Neurology is especially well positioned for opportunities to enhance clinical care and medical training
New review distills insights from studies over the past decade