Novel stimulation paradigm holds promise to mitigate side effect risks
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
Deep brain stimulation (DBS) is now a standard treatment for Parkinson disease (PD), offering hope of significant relief of debilitating motor signs for patients with advanced-stage, medically refractory disease. Current therapy relies on continuous, high-frequency stimulation of the targeted area to achieve motor benefits. However, the targeted subcortical nuclei, including the subthalamic nucleus, includes both motor and nonmotor (i.e., cognitive and behavioral) pathways. Surgical teams and clinical programmers attempt to modulate neural activity along the motor networks while limiting current spread to nonmotor areas to the extent possible.
My colleagues and I are investigating novel approaches to DBS for PD in order to identify new ways to optimize therapy delivery while furthering our understanding of PD pathophysiology and the therapeutic mechanisms of DBS.
The traditional DBS approach involves surgical implantation of a pacemaker-like device to deliver a continuous stream of high-frequency electrical pulses to either the subthalamic nucleus or the internal segment of the globus pallidus — key nodal points in the brain’s sensorimotor circuitry. Once in place, the specific parameters (e.g., amplitude) of stimulation are adjusted by a healthcare provider to maximize therapeutic benefit while avoiding stimulation-induced side effects. Once optimized, DBS typically is delivered continuously, 24 hours a day, for the rest of the patient’s life.
The physiologic mechanisms of DBS, like the pathophysiology of PD itself, remain a mystery. Theories concerning the latter have evolved from changes in mean firing rate across nodal points in the motor circuit to more recent hypotheses involving pathologically synchronized neural activity within and across those same nodal points. Accordingly, therapeutic benefit is thought to derive from further modulation of that activity, yet a precise mechanistic understanding remains elusive.
Advertisement
A well-targeted and well-programmed DBS lead typically yields outcomes comparable to the patient’s best motor benefit from dopamine replacement therapy — but without the fluctuations inherent to pharmacotherapy.
DBS is not without potential side effects, however, including those arising from slight misplacement of leads (allowing current to spread to brain regions outside the target area) and those thought to arise from current spread to nonmotor areas within the target itself. The latter may be more subtle and include cognitive or affective changes believed to be caused by stimulation interfering with “normal” neural processing. In either case, the effort to avoid such side effects may limit programming to parameters that are suboptimal for treating the patient’s motor deficits.
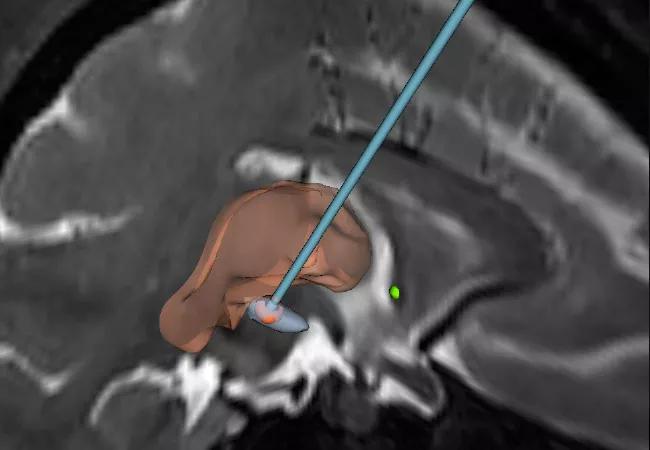
Using preclinical parkinsonian models, we are investigating a novel DBS paradigm that offers the potential to mitigate the risk of stimulation-induced side effects. The approach, termed coordinated reset DBS, was developed using computational modeling techniques by our collaborator, Peter Tass, MD, PhD, in Juelich, Germany.1 It involves using the multiple contacts of the DBS lead to deliver intermittent, pseudorandomized bursts of brief, low-intensity, spatially distributed pulse trains for the specific purpose of desynchronizing “pathological” neural oscillations (Figure 1).
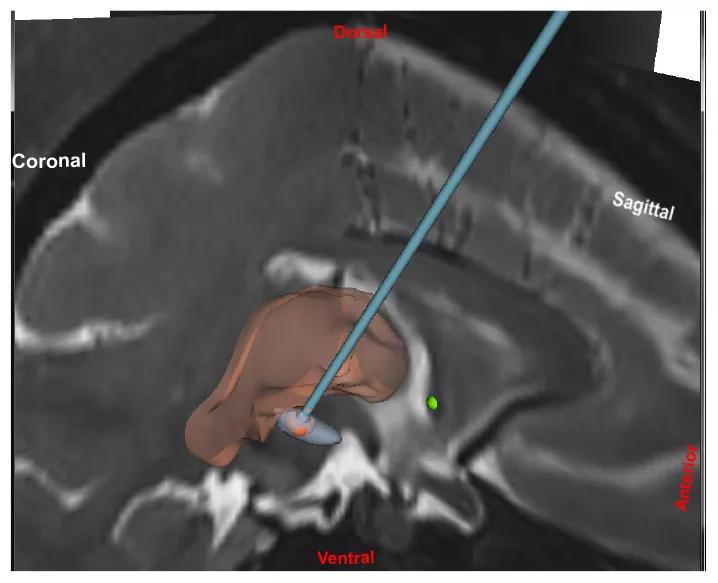
Figure 1. Representation of the coordinated reset DBS paradigm in two planes, with a view of how the DBS lead is targeted relative to coronal and sagittal MRIs.
Advertisement
An advantage of this paradigm is that its effects are achieved using lower individual pulse amplitudes, thus reducing the risk of side effects. Moreover, its desynchronizing effects are hypothesized to endure beyond treatment delivery, perhaps due to plastic changes at the synaptic level, such that intermittent therapy may yield benefit that outlasts stimulation by days or weeks. Such carryover would substantially reduce the overall duty cycle of stimulation, further limiting the potential for stimulation to interfere with cognitive or affective function.
The therapeutic potential of coordinated reset DBS is supported by multiple theoretical models and, more recently, by our own experimental data comparing coordinated reset DBS against traditional DBS of the subthalamic nucleus in terms of the magnitude and time course of their effects on motor performance in a preclinical parkinsonian model.2 In contrast to the limited carryover effects observed in response to traditional DBS, coordinated reset treatment yielded benefit that accumulated over five days of intermittent treatment (four hours a day) and subsequently persisted for over one week following therapy cessation (Figure 2).
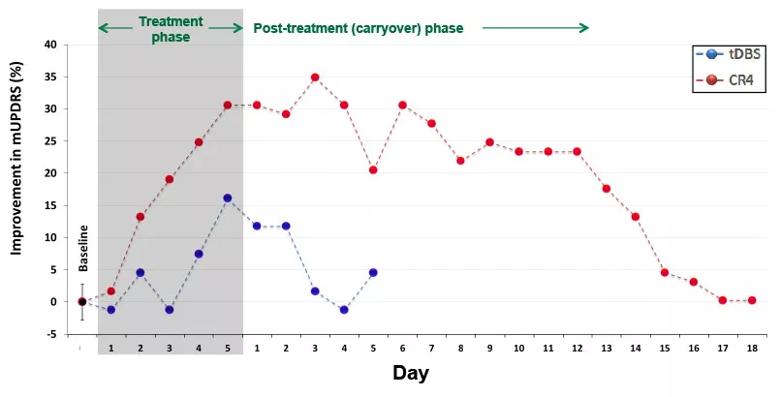
Figure 2. Daily percentage changes from baseline in parkinsonian motor severity (mUPDRS) in response to five days of traditional DBS (tDBS; blue) versus coordinated reset DBS (CR4; red). The initial composite rating score was recorded at the beginning of each daily session across the experiment. Each data point represents the OFF DBS rating recorded each morning, including that taken just before stimulation delivery each day of the five-day treatment phase (shaded gray region). In contrast to traditional DBS, coordinated reset DBS was associated with significant, cumulative improvement in the OFF DBS motor score over the five days of treatment, which persisted for more than a week after therapy cessation.
Advertisement
In subsequent work, we will further investigate the nature and time course of coordinated reset efficacy to better understand the best way to translate the approach to patients with PD while also characterizing its effects on neural activity across subcortical and cortical motor circuits. Overall, we anticipate that our data will address not only the translational potential of coordinated reset DBS but also the link between changes in neural activity in the motor circuit and motor sign manifestation in PD.
Dr. Baker is an assistant staff member in the Department of Neurosciences in Cleveland Clinic Lerner Research Institute.
Advertisement
Advertisement

New study advances understanding of patient-defined goals

Testing options and therapies are expanding for this poorly understood sleep disorder
Real-world claims data and tissue culture studies set the stage for randomized clinical testing
Digital subtraction angiography remains central to assessment of ‘benign’ PMSAH
Cleveland Clinic neuromuscular specialist shares insights on AI in his field and beyond

Findings challenge dogma that microglia are exclusively destructive regardless of location in brain

Neurology is especially well positioned for opportunities to enhance clinical care and medical training
New review distills insights from studies over the past decade