Trial aimed at improving outcomes for patients with BE and esophageal cancer now open
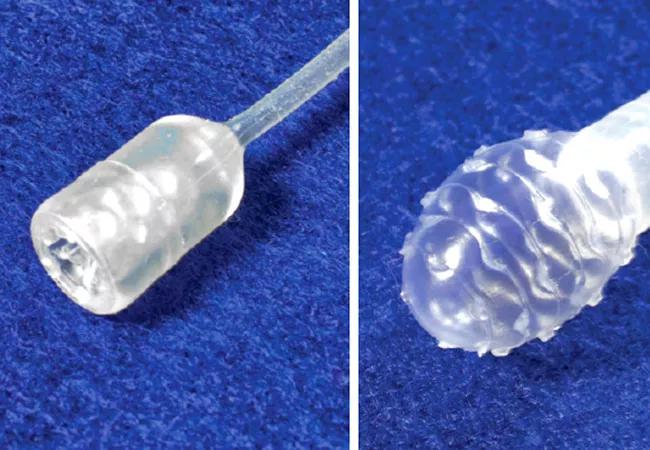
Although the rate of many common cancers has declined, the incidence of esophageal adenocarcinoma has increased greater than six-fold over the past three decades. Furthermore, the outcome of this cancer remains poor, accounting for over 1 in 50 adult male cancer-related deaths.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
Barrett’s esophagus (BE), a precursor of esophageal cancer, can be easily recognized at endoscopy; however, current clinical strategies of endoscopic screening and surveillance based on the close association of BE with chronic gastroesophageal reflux disease (GERD) in adults are woefully inadequate. Despite endoscopic screening and surveillance efforts, less than 5 percent of esophageal cancers are diagnosed in individuals with previously detected BE.
The National Institutes of Health is funding a multicenter clinical trial aimed at improving outcomes for patients with BE and esophageal cancer. Prashanthi Thota, MD, Director for Center of Swallowing & Motility Disorders at Cleveland Clinic, and study collaborators at different academic centers are testing whether a swallowable capsule can gather a sample and assay methylated DNA biomarkers to diagnose BE and esophageal cancer.
Adult white males with GERD for five years or longer are the group at the highest risk for BE and esophageal cancer, which is reflected in the study’s inclusion criteria. The study is trying to recruit subjects who have not had prior EGD and are:
OR have GERD > 5 years, Age > 40 < 85, and No prior EGD PLUS at least three of the following criteria:
If you have a patient who could benefit from this clinical trial, please email Dr. Thota at thotap@ccf.org or call at 216.444.0780.
Advertisement
Advertisement

Strong patient communication can help clinicians choose the best treatment option

ctDNA should be incorporated into care to help stratify risk pre-operatively and for post-operative surveillance

The importance of raising awareness and taking steps to mitigate these occurrences
New research indicates feasibility and helps identify which patients could benefit

Treating a patient after a complicated hernia repair led to surgical complications and chronic pain
Standardized and collaborative care improves liver transplantations
Fewer incisions and more control for surgeons

Caregiver collaboration and patient education remain critical