A veteran academic research organization finds its mission more critical than ever

A. Michael Lincoff, MD
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
No one disputes that clinical studies are necessary for medical progress, but more than a few providers and researchers believe the traditional method of evaluating drugs and devices is flawed. A central concern is that allowing industry sponsors to exclusively oversee trials of their own products opens the door for selective interpretation of data and omission of negative findings.
Cleveland Clinic considers the clinical trial process better served by the strong, impartial oversight provided by an academic research organization (ARO) in partnership with industry, and it has backed up this conviction by operating its own ARO, the Cleveland Clinic Coordinating Center for Clinical Research (C5Research), for the past quarter century.
Created in 1991, C5Research today consists of more than 100 scientific and clinical leaders from Cleveland Clinic’s Miller Family Heart & Vascular Institute who collaborate to coordinate the operation of phase 2 to 4 multicenter clinical trials for the biopharmaceutical and device industries. They also support NIH-funded and Cleveland Clinic investigator-initiated clinical research, in addition to working with other AROs and contract research organizations (CROs) to develop, implement and successfully conduct clinical trials.
“In this current atmosphere, where there is a rising degree of concern whether trial results are complete and honest, we provide credibility,” says Cleveland Clinic cardiologist A. Michael Lincoff, MD, Director of C5Research. “There are no conflicts of interest, and the trials run smoothly.”
Advertisement
AROs are not new, but just a few of them exist. C5Research was modeled after the Duke Clinical Research Institute, the granddaddy of them all. “We aren’t as big as Duke, but philosophically we are no different,” says Dr. Lincoff.
In a traditional clinical trial, an industry sponsor outsources operations to a CRO, which organizes all aspects of the trial, collects and transfers data to a database, and hands the database over to the sponsor. There is no academic oversight.
Even in an academically led trial, participating physicians must rely on industry updates and data analyses from the CRO to form their conclusions. “That’s not the best way to ensure a trial is academically rigorous and credible,” says Dr. Lincoff.
C5Research takes a different tack. From the get-go, C5Research physician leaders have their hands on every aspect of the trial. They consider the research question that needs to be addressed and design a protocol that lends itself to realistic subject recruitment and retention. The leaders help choose and educate participating sites and work closely with these sites to identify and solve any problem that might impact outcomes.
“Our physician leaders understand all details of the trial, its problems and its strengths,” Dr. Lincoff explains. “This is only possible when you are involved in day-to-day management.”
After the study period ends, the leaders take the sealed database and use the raw data to perform an accurate, impartial analysis that will hold up under scrutiny when presented at a major medical meeting or published in a peer-reviewed journal. “We vouch for these findings, because we know the study’s flaws and strengths,” says Dr. Lincoff.
Advertisement
To date, C5Research has managed over 150,000 patients in trials involving more than 1,000 participating sites. Trial sizes have ranged from fewer than 100 patients up to 24,000 patients.
Among the studies coordinated by C5Research are the trials that helped to bring abciximab and bivalirudin to market, as well as investigations of drugs to prevent progression of atherosclerosis, to modify cholesterol or its fractions, to inhibit inflammation and to prevent cardiovascular complications in patients with diabetes or obesity. “Our IV ultrasound (IVUS) trials under the leadership of Dr. Steve Nissen also established standards for how drugs affect atherosclerosis progression,” Dr. Lincoff notes.
Although the culture of AROs is rooted in cardiovascular medicine, C5Research is open to investigations in any specialty area and has provided its expertise for trials in bariatric medicine, gastroenterology, neurology and emergency medicine.
In addition to running studies, C5Research team members often collaborate on trials initiated by colleagues in institutions without AROs of their own. “Many of these physicians want to contribute to science and enjoy the camaraderie a research project involves,” says Dr. Lincoff. “They become energized by the process and are enthusiastic and confident they will obtain higher-quality data and better follow-up by partnering with us.”
Advertisement
Advertisement

A sampling of outcome and volume data from our Heart & Vascular Institute
Concomitant AF ablation and LAA occlusion strongly endorsed during elective heart surgery
Large retrospective study supports its addition to BAV repair toolbox at expert centers
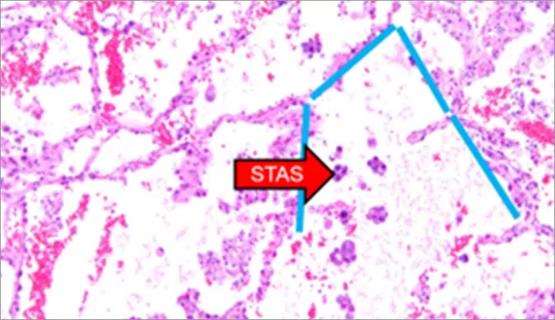
Young age, solid tumor, high uptake on PET and KRAS mutation signal risk, suggest need for lobectomy
Surprise findings argue for caution about testosterone use in men at risk for fracture
Residual AR related to severe preoperative AR increases risk of progression, need for reoperation

Findings support emphasis on markers of frailty related to, but not dependent on, age
Provides option for patients previously deemed anatomically unsuitable