Academic consortium proposes framework for regulated transparency

Good idea, but beware unintended consequences. That’s the bottom line of a new proposal from a consortium of academic cardiovascular researchers in response to calls from major medical organizations for greater transparency of clinical trial data.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
The proposal, published in the Aug. 4 issue of The New England Journal of Medicine (NEJM), dubs itself “a strategy to thoughtfully operationalize the recommendations of the International Committee of Medical Journal Editors (ICMJE) and the Institute of Medicine (IOM) for sharing clinical trial data.” The ICMJE put out its recommendations in January 2016, while the IOM recommendations came a year earlier.
The consortium of researchers — known as ACCESS CV (Academic Research Organization Consortium for Continuing Evaluation of Scientific Studies – Cardiovascular) — lauds the spirit of the ICMJE and IOM recommendations but warns that the devil is in the details.
“Full disclosure of clinical trial results has long been a cornerstone of the academic medical community’s participation in clinical trials,” says ACCESS CV member A. Michael Lincoff, MD, a co-author of the proposal in NEJM.
“The proposals by the IOM and ICMJE to provide access to primary data from clinical trials may have value for some academic research efforts, but they also carry a number of potential dangers,” adds Dr. Lincoff, Director of the Cleveland Clinic Coordinating Center for Clinical Research (C5Research) and a practicing cardiologist.
Part of the ACCESS CV paper in NEJM outlines those dangers, which include:
Advertisement
“For reasons like these, hundreds of investigators throughout the world have come together to form ACCESS CV to propose a framework that would allow sharing of data from clinical trials, but in a responsible, accountable and scientifically valid manner,” Dr. Lincoff says.
Much of the NEJM paper lays out the principles of an alternate framework proposed by ACCESS CV for sharing data from cardiovascular trials. Among the key recommendations:
The proposal’s authors identify “how to provide meaningful academic credit to the team that designed and conducted the trial” as an unresolved issue. Noting that funding will be needed to realize their proposal, the authors state that they plan to work with trial sponsors, the IOM, the ICMJE, and governmental and regulatory authorities to develop the needed data-sharing infrastructure.
Advertisement
The proposal notes that it may well apply to trials in clinical areas beyond cardiovascular disease, and Cleveland Clinic oncologist Mikkael Sekeres, MD, MS, was among a handful of noncardiovascular specialists on the ACCESS CV writing team.
“Availability and openness of trial results is fundamental to what we do across all disciplines of academic medicine,” notes Dr. Lincoff. “This additional step of complete transparency of all primary data is new. It would be beneficial in allowing unprecedented aggregation of trial results for greater statistical power to answer research questions. But it needs to be done in a way that minimizes potential risks to patients and scientific validity.”
Advertisement
Advertisement

A sampling of outcome and volume data from our Heart & Vascular Institute
Concomitant AF ablation and LAA occlusion strongly endorsed during elective heart surgery
Large retrospective study supports its addition to BAV repair toolbox at expert centers
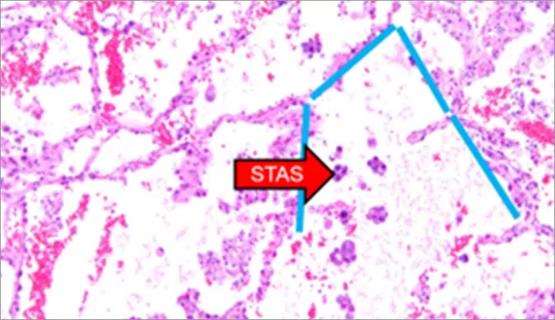
Young age, solid tumor, high uptake on PET and KRAS mutation signal risk, suggest need for lobectomy
Surprise findings argue for caution about testosterone use in men at risk for fracture
Residual AR related to severe preoperative AR increases risk of progression, need for reoperation

Findings support emphasis on markers of frailty related to, but not dependent on, age
Provides option for patients previously deemed anatomically unsuitable