Trials point to methylphenidate as good option

By Tatiana Falcone, MD, and Diana Lorenzo, MD
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
Attention deficit hyperactivity disorder (ADHD) is the most common comorbidity in pediatric epilepsy. For children with ADHD but without epilepsy, some studies have established the short-term therapeutic benefit of medication, primarily stimulants.
However, parents and providers may be reluctant to treat children with epilepsy and ADHD using stimulant medications out of concern that stimulants may precipitate seizures. Several studies in children with well-controlled seizures and ADHD suggest there is no seizure frequency increase with stimulant treatment. But most of those studies excluded children with poorly controlled seizures. Therefore, whether it is safe to use stimulant medication in children with uncontrolled epilepsy and ADHD continues to be an unanswered question.
Methylphenidate (MPH) is an effective treatment for symptoms of ADHD in children without epilepsy.1,2
Several studies have demonstrated that MPH reduces the symptoms of ADHD in children with epilepsy (CWE). However, these open trials4-6 and placebo-controlled studies7,8 have evaluated only CWE patients with well-controlled seizures. Table 1 summarizes studies, other than case report studies, conducted to date on MPH treatment of ADHD in CWE.
Feldman et al. reported that MPH improved ADHD symptoms in 7 of 10 children on the Teacher Behavior Rating Scale with no MPH-associated epileptiform changes or side effects.7 Gross-Tsur et al. demonstrated improvement of ADHD symptoms in 70 percent of patients. These authors reported no change in seizure frequency and concluded that MPH was safe and effective for seizure-free CWE. However, there were not enough subjects with active seizures in this study to actually determine the safety of MPH with respect to seizure frequency.4
Advertisement
Gucuyener et al. reported a beneficial effect of MPH and no change in seizure frequency from baseline. However, five patients on multiple anti-epileptic drugs (suggesting poor seizure control) had seizures during the study. This paper did not specify the percentage of improvement of patients’ ADHD symptoms while taking MPH or the types of side effects, although they were described as mild and transient.5
Gonzalez-Heydrich et al.’s crossover design in 33 subjects taking osmotic-release oral system (OROS) MPH found “robust” evidence that patients’ ADHD symptoms improved. However, patients’ daily seizure risk increased with increasing doses of OROS-MPH, suggesting a potential safety concern. The study revealed no serious adverse events; the most frequently reported were seizures, mainly in subjects on higher doses, and emotional lability.8
In contrast to the above studies, the 24 subjects in the Koneski et al. open-label study had experienced at least two seizures within the previous six months. These authors reported an overall improvement in ADHD symptoms in 70.8 percent of the study’s patients, with no increase in seizure frequency in 91.6 percent of patients.6
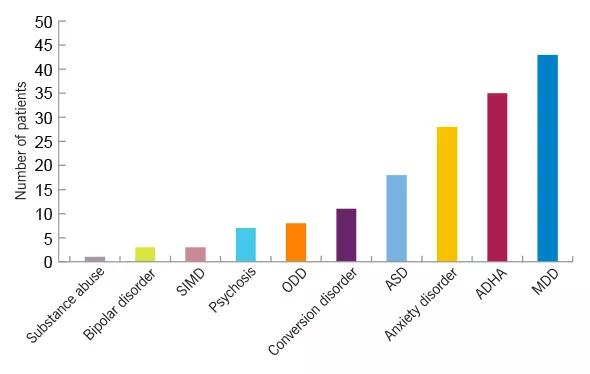
Figure 1. Psychiatric comorbidities in children and adolescents with epilepsy. In a retrospective study of 116 children 18 years old and younger who were screened in the pediatric epilepsy monitoring unit, ADHD was the second most frequent comorbidity in youths with epilepsy. SIMD = substance-induced mood disorder; ODD = oppositional defiant disorder; ASD = autism spectrum disorder; MDD = major depressive disorder. (Falcone T, Klaas P, Kotagal P. Psychiatric comorbidities in children and adolescents undergoing epilepsy surgery. Presented at the 2009 American Epilepsy Society annual meeting.)
Advertisement
A 12-week prospective trial of atomoxetine (ATX), a norepinephrine reuptake inhibitor, in 17 children and adolescents with epilepsy showed significant improvement of ADHD symptoms. Side effects were sedation, loss of appetite and nausea. One patient had an increase in the number of seizures.10
A retrospective study examined 25 epilepsy patients with stimulant-resistant ADHD treated with ATX. None of the patients’ seizures worsened. Seventeen patients discontinued ATX due to inadequate response, increased irritability, emerging psychotic-like symptoms, decreased appetite and tremors.3
The antidepressant bupropion is contraindicated in the treatment of CWE since it is the only medication used in ADHD treatment with a well-documented risk of seizures.8
Clonidine and guanfacine, both sympatholytic medications, are used to treat ADHD, but we are not aware of any prospective, controlled trials of clonidine or guanfacine in children with ADHD and epilepsy.
On the basis of the research we reviewed, there is evidence that MPH can provide safe, effective treatment of ADHD in children with well-controlled epilepsy. Initial doses should be low and short-acting and should be accompanied by close monitoring for seizures.
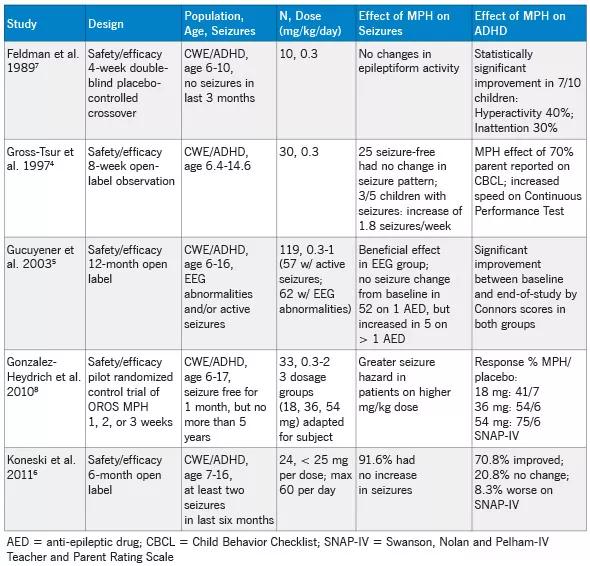
Table 1. Prospective studies of MPH in children with epilepsy.
Dr. Falcone is a child and adolescent psychiatrist at Cleveland Clinic and Assistant Professor of Medicine at Cleveland Clinic Lerner College of Medicine. She is the Principal Investigator for Project CARE (Coordination, Access, Resources, Education) 4 Epilepsy, a three-year Health Resources and Services Administration grant to enhance services, including mental health services, for children with epilepsy.
Advertisement
Dr. Lorenzo is a psychiatry resident at Cleveland Clinic.
Advertisement
10. Hernandez J, Barragan E, Garza S. Efficacy of Atomoxetine Treatment in Children With ADHD and Epilepsy. Presented at the Annual Meeting of the International League Against Epilepsy, Paris, Aug. 18-Sep. 1, 2005.
Advertisement

New study advances understanding of patient-defined goals

Testing options and therapies are expanding for this poorly understood sleep disorder
Real-world claims data and tissue culture studies set the stage for randomized clinical testing
Digital subtraction angiography remains central to assessment of ‘benign’ PMSAH
Cleveland Clinic neuromuscular specialist shares insights on AI in his field and beyond

Findings challenge dogma that microglia are exclusively destructive regardless of location in brain

Neurology is especially well positioned for opportunities to enhance clinical care and medical training
New review distills insights from studies over the past decade