Q&A with lead author of the GATE trial

For the first time, a generic version of a disease-modifying therapy for multiple sclerosis (MS) has been studied in a phase 3 trial — and it was found to be equivalent in efficacy, safety and tolerability to its brand-name predecessor. That was the upshot of the GATE trial, published recently in JAMA Neurology, which compared a generic form of glatiramer acetate to the costly branded version of the compound, known as Copaxone®.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
GATE’s lead author, Jeffrey Cohen, MD, Director of Cleveland Clinic’s Mellen Center for Multiple Sclerosis Treatment and Research (pictured above), recently fielded questions from Medscape about this novel study as part of a Medscape-Cleveland Clinic editorial collaboration. The resulting Q&A, available here, provides Dr. Cohen’s perspectives on the study’s findings and their implications for enhanced affordability and accessibility of effective therapies for MS.
Advertisement
Advertisement

New study advances understanding of patient-defined goals

Testing options and therapies are expanding for this poorly understood sleep disorder
Real-world claims data and tissue culture studies set the stage for randomized clinical testing
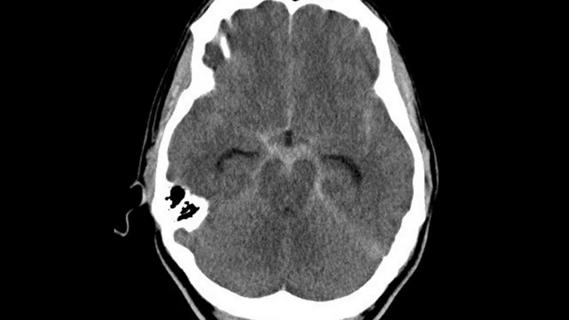
Digital subtraction angiography remains central to assessment of ‘benign’ PMSAH
Cleveland Clinic neuromuscular specialist shares insights on AI in his field and beyond

Findings challenge dogma that microglia are exclusively destructive regardless of location in brain

Neurology is especially well positioned for opportunities to enhance clinical care and medical training
New review distills insights from studies over the past decade